An 11-year-old boy with a posterior fossa tumor
BOX 1. Virtual glass slide
Access at https://isn-slidearchive.org/?col=ISN&fol=Archive&file=BPA-24-10-CIR-279.tiff
1 CASE PRESENTATION
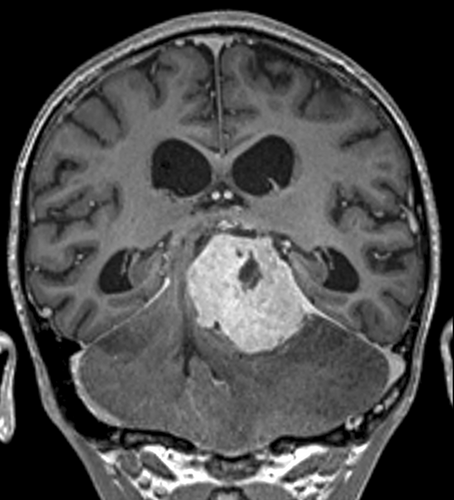
An 11-year-old boy was referred to our institution from a resource-limited country with a history of hydrocephalus secondary to a posterior fossa tumor. Magnetic resonance imaging (MRI) revealed a large extra-axial neoplasm located at the tentorium, measuring 5.1 × 4.2 × 4.0 cm (Figure 1), causing marked distortion of the adjacent structures and transtentorial herniation. The lesion was strongly enhancing, associated with massive vasogenic edema. Microsurgical excision of the lesion was performed by a supracerebellar infratentorial approach, obtaining a gross total resection. After recovery, the boy returned to his country, where he has been followed with periodical computed tomography scans. He remains free of disease 58 months from surgery (Box 1).
2 FINDINGS
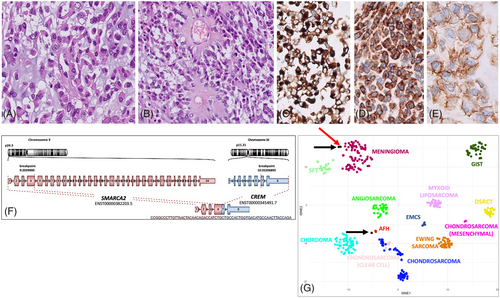
The lesion consisted of epithelioid and rhabdoid cells arranged in a chordoid pattern within a myxoid stroma (Figure 2A) with foci of collagen deposition sometimes featuring amianthoid fibers (Figure 2B). Solid sheets of rhabdoid and epithelioid cells with focal clear cell changes were also present. Mitotic figures were infrequent (1/1.96 mm2). There was a sparse lymphoplasmocytic inflammatory infiltrate at the periphery. The possibility of a chordoid meningioma was first considered, and the expression of epithelial membrane antigen (EMA) seemed to support this hypothesis. However, given the rarity of this meningioma subtype and the lack of a specific marker, an extensive immunohistochemical panel was performed: the diffuse desmin expression, combined with focal CD99 and D2-40, prompted us to consider an intracranial mesenchymal tumor (IMT) with FET::CREB fusion (Figure 2C–E). RNA sequencing (Agilent) demonstrated the presence of a SMARCA2::CREM transcript (Figure 2F). No pathogenic/likely pathogenic variants were identified (TSO500). CGH/SNP array revealed only a mosaic 12p trisomy. Genome-wide DNA methylation profiling classified the lesion as meningioma (score 0.98), subclass 3 (score 0.93) according to the Heidelberg Brain Tumor Classifier v12.5. t-distributed stochastic neighbor embedding (t-SNE) analysis was performed using selected Deutsches Krebsforschungszentrum (German Cancer Research Center) (DFKZ) reference classes and two previously characterized IMT cases, both harboring an EWSR1::CREM fusion. The current case clustered with one of the IMT and was close to meningiomas, whereas the other IMT was near angiomatoid fibrous histiocytomas (Figure 2G).
3 FINAL DIAGNOSIS
IMT harboring a SMARCA2::CREM fusion.
4 DISCUSSION
We describe a rare example of a pediatric posterior fossa IMT with a SMARCA2::CREM fusion. A case with an identical fusion transcript has been reported in the parietal lobe of a 40-year-old man with a 15-year history of multiple recurrences of a “chordoid meningioma” [1]. These cases share a striking chordoid morphology. IMT generally shows two main patterns: (i) stellate/spindle cells and abundant myxoid stroma preferentially characterizing CREB1/CREM-rearranged cases and (ii) epithelioid/rhabdoid cells and mucin-poor stroma frequently associated with ATF1 rearrangement [2]. Curiously, both SMARCA2::CREM IMT displayed a combination of these morphological patterns, a feature per se not specific, given that a similar mixture is encountered in a minority of IMT cases with canonical FET::CREB rearrangements [2]. As a diagnostic clue, both cases expressed desmin. Despite remarkable similarities, the two cases differed in terms of mitotic activity and Ki67 index, which were low in ours and elevated in the previous case. Their clinical course also seems quite different: while our patient has not shown any sign of disease nearly 5 years from surgery, the other experienced multiple recurrences, despite the initial total resection and radiotherapy, and died of lung metastases 15 years from the original diagnosis [1].
DNA methylation analysis shows limited utility in IMT diagnosis, as the large majority of cases yield a no match result in the DKFZ Brain Tumor Classifier [2, 3]. In line with this notion, the previous SMARCA2::CREM IMT proved no match. Notably, our case aligned with meningioma, indicating that DNA methylation analysis was misleading, definitely an issue considering the frequent EMA positivity of these tumors. The possibility of an IMT should be considered, based on the morphological features and addressed with the appropriate immunostains, especially desmin. These findings may in part reflect the epigenetic heterogeneity of this entity, suggested by two studies, which, despite some differences in the results, independently showed that IMT does not form a single methylation cluster on the t-SNE-analysis plot [2, 3].
SMARCA2 rearrangement has been reported rarely in other tumors, including an extraskeletal myxoid chondrosarcoma and a salivary gland hyalinizing clear cell carcinoma harboring a SMARCA2::NR4A3 and a SMARCA2::CREM fusion, respectively [1], where the canonical 5′ partner of the driver fusion is EWSR1, pointing to the interchangeable role of SMARCA2 and EWSR1 in the chimeric protein.
The identification of IMT cases driven by the rearrangement of CREM with a non-FET partner gene hints at a major contribution of the CREB family genes to IMT biology and should prompt reassessment of the nomenclature for IMT in the next WHO central nervous system tumors classification.
AUTHOR CONTRIBUTIONS
Study conception: SR, RA, EM, GDV. Acquisition of data: SR, SB, VA, SP, CD, SG, EM, IG, GSC, AM, AC, AF. Analysis and interpretation of data: SR, SB, GSC, IG, CT, EM, VA, SP, GSC. Drafting of manuscript: SR, GDV. Critical revision: AM, GSC, EM, VA, RA. All authors approved the final version of the manuscript to be published.
ACKNOWLEDGMENTS
This work was supported by the Italian Ministry of Health with current research funds. We wish to thank Mr. Gabriele Bacile for iconography preparation.
CONFLICT OF INTEREST STATEMENT
None of the authors declare conflicts of interest.
ETHICS STATEMENT
Written consent for the publication of the case details was given by the patient's parents.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




