Brain-derived textiloma post glioblastoma resection and application of oxidized regenerated cellulose: A pilot, bedside-to-bench, translational study
Joshua A. Kra, Christopher Markosian, and Fenny H. F. Tang contributed equally to this study.
Daniela I. Staquicini, Renata Pasqualini, and Wadih Arap jointly supervised this study.
Abstract
Oxidized regenerated cellulose (ORC; marketed as Surgicel® and Tabotamp®) is routinely used as an intraoperative hemostatic agent. Rarely, residual ORC has been associated with a foreign body reaction generating cystic or granulomatous lesions (i.e., textilomas) at the surgical site. Here, we report a bedside-to-bench, translational report of an intracranial mass after neurosurgical resection of glioblastoma with ORC application. As part of patient care, we performed magnetic resonance imaging and histopathological analysis of the mass. We then performed in vitro studies to evaluate the effect of ORC on cytokine production and viability of BV-2 murine microglial cells by using quantitative PCR along with live cell microscopy and crystal violet staining, respectively. Magnetic resonance imaging demonstrated a recurrent mass pressing on the adjacent right ventricle, which was removed in a second surgery for diagnostic and therapeutic purposes. Unexpectedly, histopathological examination of the resected mass revealed abundant ORC arising from the site with inflammation, microglial activation, and collagenization. Mechanistically, we show an ORC-induced modest increase in inflammatory cytokines with a subsequent decrease in microglial cell viability. These findings suggest that ORC may mediate microglial immune response and viability, and serve to raise awareness and guide interpretation of post-treatment surveillance imaging findings in the instance of foreign body reaction.
1 INTRODUCTION
Management of patients with high-grade glioma generally involves upfront maximal safe surgical resection [1]. For most patients, postoperative treatment will typically involve concurrent chemoradiotherapy consisting of temozolomide and standard or hypofractionated radiation therapy for 6 weeks [2, 3]. Serial magnetic resonance imaging (MRI) follow-up studies are used to detect potential progression of the tumor [1] and surgical complications, such as abscesses or even tissue necrosis occurring post-radiation therapy [4]. Over the past two decades, glioblastoma patients usually commence standard adjuvant temozolomide on or about 4 weeks after the completion of concurrent radiotherapy, along with continuous monitoring for recurrence with serial brain imaging studies [2, 3].
Oxidized regenerated cellulose (ORC), a Food and Drug Administration- and European Medical Agency-approved hemostatic agent (marketed as Surgicel® and Tabotamp®), is commonly used as a hemostatic agent in surgical procedures given its ability to be resorbed in operative cavities [5, 6]. However, in certain rare cases, hemostatic agents used in intraoperative procedures can unexpectedly trigger a foreign body reaction resulting in either cystic or granulomatous lesions (i.e., textilomas) that may resemble tumor recurrence on MRI, and could lead to serious postoperative complications [7, 8]. To date, there are at least 19 previous reports of adverse reactions to the use of ORC [7, 9-26], of which 12 involve the cranial cavity [7, 9-11, 15, 19-21, 23-26]. However, the underlying molecular and/or cellular mechanism(s) for this phenomenon has not as yet been elucidated. Here we report a bedside-to-bench, translational case study to gain mechanistic insight into this uncommon, untoward effect of ORC.
2 MATERIALS AND METHODS
2.1 Histopathological analyses
Standard pathological analysis of tissues with hematoxylin and eosin (H&E) staining was used. The Masson's trichrome procedure was also performed. Immunostainings for glial fibrillary acidic protein (GFAP), ionized calcium-binding adapter molecule-1 (IBA-1), cluster of differentiation 68 (CD68), CD3, and CD20 were performed as indicated. Tissue sections were imaged with a DM2000 dual viewing microscope (Leica Camera AG; Wetzlar, Germany) attached to a VIEW4K camera (Microscope Central; Feasterville, PA, USA) or a BX63 automated fluorescence microscope (Olympus Life Science; Waltham, MA, USA) attached to a DP27 microscope digital camera (Olympus Life Science).
2.2 Prognostic biomarkers
Standard clinical pathology assays for the biomarkers, isocitrate dehydrogenase (IDH) mutational status (i.e., wild-type vs. mutant) and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status (i.e., unmethylated vs. methylated), were performed.
2.3 Microglial cell line and tissue culture
BV-2 is a microglial cell line derived from C57/BL6 mice, immortalized by v-raf/v-myc carrying J2 retrovirus [27]. BV-2 microglial cells (AcceGen; Fairfield, NJ, USA) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were grown as a monolayer at 37° C and 5% CO2, and tested for Mycoplasma contamination prior to experiments by using the MycoAlert Mycoplasma Detection Kit (Lonza; Basel, Switzerland).
2.4 Transwell cell migration assays
BV-2 microglial cells were seeded at 105 cells in 500 μL of media in the lower chamber of a 24-well transwell plate (Corning; Glendale, AZ, USA) overnight (ON). On the next day, the medium was replaced, and the cells were incubated with sterile 1.6 cm2 square strips of Surgicel® (Ethicon; Raritan, NJ, USA) placed at the top of an 8.0 μm pore polycarbonate membrane insert (Corning) with 100 μL of media. Cells incubated with 1 μg/mL of lipopolysaccharides (LPS) from Klebsiella sp. (Sigma-Aldrich; St. Louis, MO, USA) in 100 μL of media in the insert served as the positive control for microglial activation as described [28, 29]; cells incubated with medium-only served as the negative control.
2.5 Quantitative gene expression
BV-2 murine microglial cells incubated with Surgicel®, LPS, or medium-only for 2 h were rinsed with phosphate-buffered saline (PBS) and used for total RNA extraction by using the PureLink RNA Mini Kit (Invitrogen; Waltham, MA, USA). cDNA synthesis was performed with the ImProm-II Reverse Transcription System (Promega; Madison, WI, USA). Gene expression analysis was performed by using the TaqMan Fast Advanced Master Mix and fluorescein (FAM) dye-labeled TaqMan MGB probes (Applied Biosystems; Waltham, MA, USA) in a QuantStudio 5 (Applied Biosystems), as depicted in Table 1. Murine 18S ribosomal RNA served as an endogenous control.
| Gene symbol | Gene name | TaqMan assay ID |
|---|---|---|
| Il1b | Interleukin-1b | Mm00434228_m1 |
| Il6 | Interleukin-6 | Mm00446190_m1 |
| Il10 | Interleukin-10 | Mm01288386_m1 |
| Il12b | Interleukin-12b | Mm01288989_m1 |
| Nos2 | Nitric oxide synthase-2 | Mm00440502_m1 |
| Rn18s | 18S ribosomal RNA | Mm04277571_s1 |
| Tgfb1 | Transforming growth factor b-1 | Mm01178820_m1 |
| Tnf | Tumor necrosis factor | Mm00443258_m1 |
2.6 Live cell microscopy
Cells incubated with Surgicel®, LPS, or medium-only control were monitored and imaged with a CKX53 inverted microscope (Olympus Life Science) attached to a DP74 color fluorescence camera (Olympus Life Science).
2.7 Crystal violet staining
After 24 h, cells incubated with Surgicel®, LPS, or medium-only were rinsed with PBS and fixed with PBS containing 4% paraformaldehyde for 10 min at room temperature (RT). After additional washes, the cells were stained with an aqueous solution consisting of 0.5% crystal violet and 50% methanol for 10 min at RT. By submerging the plate in 4 L of double-distilled water, the cells were washed several times until the soluble dye was completely removed. The plate was allowed to air-dry ON at RT. The crystal violet was dissolved in 70% ethanol and allocated to a 96-well plate. Optical density was measured at an absorbance wavelength of 570 nm on a Varioskan LUX Multimode Microplate Reader (Thermo Scientific; Waltham, MA, USA) on a standard calibration curve.
2.8 Statistics
Relative-fold change in gene expression was calculated by applying the formula 2−ΔΔCT. Data were plotted and analyzed by using GraphPad Prism 9. Statistical tests included one-way ANOVA and post hoc Tukey's multiple comparisons test (with multiplicity-adjusted p-values), where p < 0.05 was deemed statistically significant.
3 RESULTS
3.1 Glioblastoma patient and clinical course
A male in his 60s with a past medical history of arterial hypertension and type 2 diabetes mellitus was admitted to the hospital with a new-onset generalized tonic–clonic seizure. After recovery, physical examination was deemed normal with a nonfocal neurological examination, and a Karnofsky Performance Status of 100%. Workup at the initial presentation included MRI of the brain, which revealed a 3 cm enhancing mass in the white matter of the right temporal lobe (Figure 1A). The patient underwent a right temporal craniotomy with gross total resection of the lesion. Pathological assessment revealed glioblastoma with wild-type IDH and unmethylated MGMT promoter, both biomarkers of poor outcome [1]. Postoperative MRI confirmed successful resection of the tumor (Figure 1B). After recovery, the patient was treated with concurrent oral chemotherapy (temozolomide 75 mg/m2 daily for 6 weeks) plus radiation therapy (60 Gy in 30 daily fractions) as standard of care [2, 3].
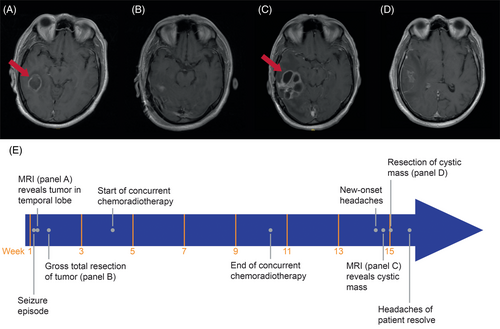
Four weeks after completion of concurrent therapy, but prior to starting oral adjuvant temozolomide, the patient complained of worsening right-sided headache characterized as constant and nonradiating, and rated as eight out of 10 in severity. This new symptom was persistent despite ongoing dexamethasone taper. Physical examination did not reveal any focal neurological deficits. A follow-up MRI of the brain demonstrated a new multilobulated cystic lesion with peripheral enhancement and increased surrounding vasogenic edema at the surgical bed (Figure 1C). Other radiographic observations included mass effect on the adjacent right lateral ventricle with effacement of the occipital and temporal horn, compression of the third ventricle, and a right-to-left midline shift. These findings were concerning for tumor recurrence or possibly post-radiation necrosis. Repeat right temporal craniotomy and resection of the cystic mass were performed in the patient for definitive tissue-based diagnosis and therapeutic symptom relief. Postoperative MRI following the second surgery revealed successful removal of the cystic mass (Figure 1D). He recovered well postoperatively, resulting in improvement and then resolution of his severe headaches along with decreased mass effect on the right ventricle. A complete timeline of disease progression and treatment history is summarized in Figure 1E.
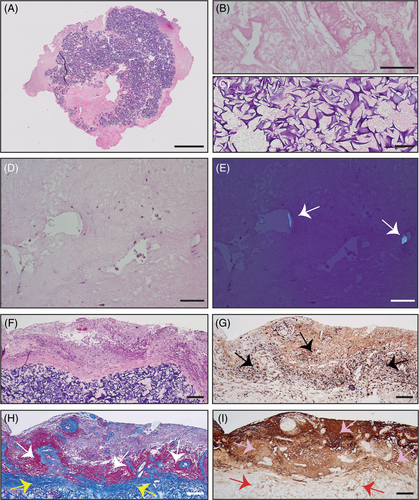
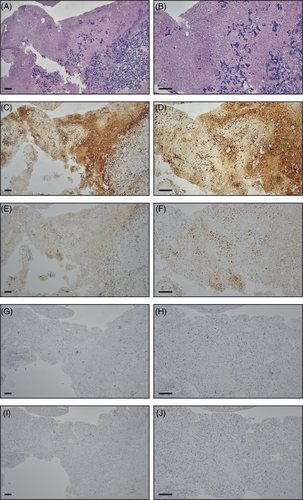
Three specimens were obtained in aggregate from the second surgery and approximately measured 5 cm × 2.5 cm × 2 cm in total. Histopathological examination of the cystic mass sample via H&E staining revealed primarily abundant foreign material (estimated to comprise 60%–75% of the shown specimen, or 40%–50% of all specimens combined), surrounded by foci of residual tumor and reactive changes (Figure 2A–E). Moreover, progressive disease (i.e., tumor growth) or treatment-induced tumor necrosis (often seen with tumor pseudo-progression because of radiation) was not observed, suggesting that cystic mass formation was most likely induced by ORC remnants from the tumor resection. In addition to H&E (Figure 2F), other stainings revealed microglial activation (IBA-1, Figure 2G), collagenization (Masson's trichrome, Figure 2H), and a high concentration of glial cells (GFAP, Figure 2I) surrounding the ORC remnants. Despite the high sensitivity of IBA-1 for microglia, it may not adequately distinguish this cell type from infiltrating macrophages. Hence, comparative staining was subsequently performed in the cystic mass and surrounding brain tissue with H&E (Figure 3A,B), IBA-1 (Figure 3C,D), and CD68 (Figure 3E,F), which is prominent in infiltrating macrophages but lower in microglia. IBA-1-positive cells were found to be more abundant than CD68-positive cells within the ORC and surrounding brain. Furthermore, few CD3-positive T-cells were present in the perivascular and parenchymal regions of the surrounding brain (Figure 3G,H), and CD20-positive B-cells were exceedingly rare (primarily in the perivascular regions) (Figure 3I,J). Because of (i) the absence of an obvious foreign body giant cell reaction that is often appreciated in postoperative specimens, and (ii) the observed marked staining of IBA-1, we speculate that a robust inflammatory reaction was driven by microglia, not giant cells, in the described case.
3.2 Bedside-to-bench, translational studies
3.2.1 Effect of ORC on cytokine production
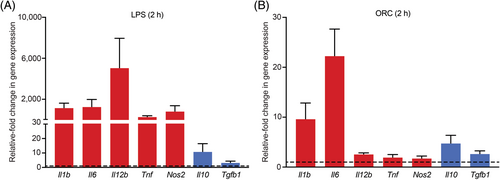
To gain mechanistic insight into this untoward clinical effect of ORC, we first designed and optimized the microglial tissue culture conditions by placing ORC inside of the porous membrane inserts in a transwell system with BV-2 microglial cells cultured in the bottom chamber. Given that microglial cells are resident macrophages of the central nervous system (CNS), we evaluated a repertoire of pro- and anti-inflammatory cytokines induced in the presence of ORC and its potential effects on cell viability. For this adapted functional assay, LPS and medium-only served as positive and negative controls, respectively. As expected, LPS (positive control) effectively induced potent induction of predominantly pro-inflammatory cytokines by microglial cells in a non-specific manner (Figure 4A) [28, 29]. Notably, we found that ORC modestly increased the production of key pro-inflammatory cytokines involved in microglial activation (Figure 4B). Specifically, ORC increased the production of interleukin (IL)-6 (>20-fold), IL-1β (~10-fold), and IL-10 (~5-fold) after 2 h of incubation. As a potential sign of biological specificity, the impact of ORC on the production of other inflammatory cytokines such as IL-12β, tumor-necrosis factor alpha, nitric oxide synthase-2, and transforming growth factor beta-1 (TGFβ1) was less remarkable. These findings reveal that ORC induces pro-inflammatory cytokine production in microglial cells in vitro, which may potentially recapitulate the mechanism of acute inflammation and/or cellular recruitment at the site of deposition in vivo. Of interest, IL-6 and IL-1β are among the main inflammatory cytokines centrally associated with microglial activation and inflammatory cell-death mechanisms with effects on cell viability [30].
3.2.2 Effect of ORC on cell viability
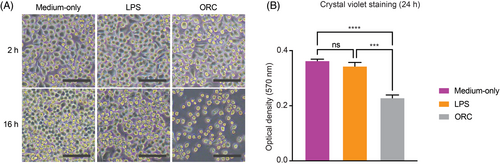
We next sought to assess the impact of ORC on cell viability considering the acute release of pro-inflammatory cytokines by microglia and its previously reported cytotoxicity. Its impact on microglia has not as yet been fully assessed in the literature to our knowledge. Over a time course, the morphology of microglia at serially fixed time points appeared to be consistently affected by ORC. Indeed, as shown in Figure 5A, microscopic images of live microglial cells incubated with ORC for 2 h and 16 h indicate progressively lower cell density when compared to cells in medium-only (negative control). Though a decrease in cell density was also observed for LPS treatment, the morphological cellular change is distinct visually compared to that for ORC treatment. Most of the microglial cells exposed to ORC exhibited cell shrinkage and loss of their branching structure after 16 h, reflecting decreased viability. Indeed, crystal violet staining followed by quantitative optical density (OD570) measurement confirmed a notable decrease in microglial cell viability incubated with ORC for 24 h. The same effect was not observed in cells incubated with LPS or medium-only (Figure 5B), data potentially indicative of specificity. Despite the marked inductions of pro-inflammatory cytokines to a degree much greater than those of cells in ORC, microglial cells in LPS at 24 h did not demonstrate a similar change in viability compared to cells in medium-only. These translational findings indicate that ORC induces a decrease in proliferation and/or viability of microglial cells, suggesting that this hemostatic agent might indeed interfere with the viability and immune response of microglia and potentially recruit macrophages at operative intracranial sites.
4 DISCUSSION
A commonly used material across surgical specialties for hemorrhage control, ORC stimulates coagulation by promoting blood clotting and sealing of anastomotic leaks. As an absorbable agent, ORC transforms into a gelatinous mass when it contacts fluids, lowers the pH of the surrounding tissue, and serves as a source for platelet adhesion and aggregation that facilitates the clotting process [6, 31]. Previous reports have described relatively uncommon but severe and unpredictable adverse reactions to ORC, including the presence of space-occupying granulomas and pathological findings of infiltrating giant cells, in the clinical setting (Table 2) [7, 9-24, 26]. Despite the exhaustive number of incidents noted in the literature, the underlying mechanisms of foreign body reaction or granuloma formation as a result of residual ORC in tumor resection cavities remain poorly understood. In the laboratory correlates (Table 2), cell death in astrocytes, neurons, Schwann cells, and even lung adenocarcinoma cells has been observed in the presence of ORC in vitro [32, 33]. MRI or computed tomography findings can include cystic lesions, possibly with mass effect, that resemble abscesses or residual tumors [15, 20, 21], which can interfere with the accuracy of imaging analysis in postoperative evaluation. Concordantly, an in vitro study demonstrated that ORC affects the longitudinal relaxation time and hence can disrupt the interpretation of early postoperative MRI scans by mimicking residual tumor [34]. Given the relative paucity of case reports and correlative laboratory studies of these undesirable side-effects of ORC—particularly in the CNS (n = 12 studies reported) [7, 9-11, 15, 19-21, 23-26]—its impact on microglia, the key immune cells of the CNS, has not been thoroughly explored.
| Authors | Year | Journal | Reference | Study type | No. of patients | Neurosurgical procedure |
|---|---|---|---|---|---|---|
| Agarwal et al. | 2010 | Urology | [14] | Case description | 1 | No |
| Agudelo-Arrieta et al. | 2023 | Interdiscip Neurosurg | [26] | Case description | 1 | Yes |
| Buckley & Broome | 1995 | Br J Neurosurg | [21] | Case description | 1 | Yes |
| Capozza et al. | 2016 | IrJNS | [24] | Case description | 1 | Yes |
| Cormio et al. | 2016 | Oncol Lett | [16] | Case description | 1 | No |
| Gao et al. | 2002 | Int J Gynecol Pathol | [12] | Case description | 1 | No |
| Hara et al. | 1996 | Acta Neurochir | [23] | Case description | 1 | Yes |
| Hernandez-Bonilla et al. | 2019 | Cancer Cytopathol | [18] | Case description | 16 | No |
| Ito et al. | 1989 | Acta Neurochir | [9] | Case description | 3 | Yes |
| Kothbauer et al. | 2001 | J Neurosurg | [11] | Case description | 3 (2 w/ ORC) | Yes |
| Leisz et al. | 2020 | Materials | [32] | Laboratory investigation | Not applicable | Not applicable |
| Lin et al. | 2014 | World J Surg Oncol | [15] | Case description | 1 | Yes |
| Loh et al. | 2022 | J Neurosurg Case Lessons | [19] | Case description | 1 | Yes |
| Lu et al. | 2022 | Mater Today Bio | [33] | Laboratory investigation | Not applicable | Not applicable |
| Ribalta et al. | 2004 | Arch Pathol Lab Med | [7] | Case description | 5 (2 w/ ORC) | Yes |
| Sandhu et al. | 1996 | Br J Neurosurg | [10] | Case description | 2 | Yes |
| Singh et al. | 2016 | Indian J Radiol Imaging | [17] | Case description | 1 | No |
| Somani et al. | 2006 | Surgery | [13] | Case description | 1 | No |
| Spiller et al. | 2001 | J Neurosurg | [34] | Laboratory investigation | Not applicable | Not applicable |
| Staglianò et al. | 2018 | Quant Imaging Med Surg | [25] | Case description | 3 | Yes |
| Wang and Chen | 2013 | Oncol Lett | [22] | Case description | 1 | No |
| Young et al. | 1993 | AJR Am J Roentgenol | [20] | Case description | 5 | Yes |
Here we report a case of cystic mass containing residual ORC that developed in the right temporal lobe of a patient following glioblastoma resection, producing unusual symptomatology (new-onset, severe headaches), which required an additional neurosurgical procedure. During the initial surgery, a double layer of ORC had been applied (in comparison to the customary single layer) within the tumor resection cavity because of greater concern for bleeding. This provides a plausible explanation as to why foreign body reactions to ORC have largely been described in the context of tumor resection throughout the literature, wherein greater-than-usual amounts of ORC may be utilized to control increased bleeding of highly vascularized tissue [35]. Given the unexpected symptoms, imaging, and histopathology, we performed a series of in vitro experiments with the BV-2 murine microglial cell line to investigate the effects of ORC in a reductionist cellular model in vitro. Because of the potential association of ORC with a rare yet unpredictable and deleterious neuroinflammatory response, it is critical to include ORC-induced foreign body reaction, or textiloma, in the differential diagnosis in the case of a new brain lesion that is detected via CNS imaging studies [8].
Previous reports have shown giant cell infiltration in ORC-induced granulomas [11, 12, 15, 18, 19, 22] and explicit evidence of macrophages in the case of another hemostat (i.e., absorbable collagen)-induced granuloma [7]. In the correlative studies reported in this initial translational work, we found microglial activation and collagenization around the residual ORC on histopathological analysis of the cystic mass sample. Moreover, our findings in microglial cells indicate that ORC modulates the local immune response while also affecting cell viability, supporting inflammation and recruitment of cells to stimulate a foreign body reaction. Indeed, microglia, which are normally found in a branched state, transform into an amoeboid shape upon activation [36] to induce a foreign body reaction over the course of a mere few hours [37]. This activation process is generally complex and associated with an adjustment in the interplay of released pro-inflammatory and anti-inflammatory cytokines [38]. In the reductionist experimental conditions tested here, microglial cells exposed to ORC demonstrated marked but likely specific morphological alterations (surprisingly, even more so than LPS) by 16 h; most microglial cells adopted an amoeboid, circular shape consistent with activation and subsequent cell death. These experimental observations were further confirmed with the serial quantification of cell biomass at 24 h showing a notable decrease in the viability of cells incubated with ORC, to an extent greater than non-specific bacterial LPS. These translational findings suggest potentially unpredictable inflammatory attributes induced by this clinically approved hemostatic material; however, further studies will clearly be required to determine the mechanisms of induced cell death and the chronic inflammatory response associated with ORC.
Our initial study has several limitations. First, the apparent rarity of this complication precludes epidemiological studies, although one might perhaps speculate that this neurosurgical condition could be missed, under-diagnosed, or under-reported. Second, we used a murine microglial cell line, BV-2, in the mechanistic studies in vitro. Based on previous studies, this particular cell line does not fully recapitulate primary microglia; limitations include the absent expression of a TGFβ-dependent [39] or LPS-triggered microglial signature [40], reduced expression of pro-inflammatory genes and cytokine signaling in the presence of LPS [41], limited TGFβ signaling and decreased chemotaxis in response to the chemoattractant C5a [41], and variability of pro-inflammatory cytokine protein expression and release when activated by LPS [42]. However, BV-2 cells retain the fundamental aspects of the primary microglial response involved in inflammation and express, albeit to a lesser degree, approximately 90% of induced genes as primary microglia in response to LPS [41, 43, 44]. Our findings in BV-2 primarily serve as a laboratory correlate with our clinical observations in this pilot study, hence similar experiments with primary microglia or even a human microglial cell line, such as HMC3, can be performed to confirm cross-species results and more accurately quantify gene expression changes in a future study. Third, immortalized cells do not fully capture the complex native environment of the brain, including the interplay with other cell types (i.e., neurons, astrocytes, lymphoid cells, and residual tumor cells). Fourth, mRNA expression levels do not necessarily equate to protein levels, and hence additional experimental strategies are necessary to confirm that IL-6 and IL-1β are indeed present in higher levels upon microglial exposure to ORC. Fifth, activated microglia upregulate pro-inflammatory cytokines in response to different pathological processes, including foreign body reactions [45]. Additional comparative studies examining the microglial inflammatory response to various hemostatic or nonhemostatic agents in the form of textiles and other materials (e.g., powders) used intraoperatively may be valuable to differentially assess cytokine profiles and cell viability. Relevant nonimmune cell types, such as glioblastoma cells, are also likely to experience reduced viability in the presence of ORC, similar to lung adenocarcinoma cells [33]. Finally, future studies in vivo in animal models of CNS tumors may be required to confirm our hypothesis, and ultimately to be able to predict, anticipate, or prevent these untoward effects of ORC in human glioblastoma patients. For example, biodegradable anti-inflammatory nanoparticles have been used successfully in an in vivo animal model to attenuate pro-inflammatory cytokine production in response to hemostatic gelatin in the brain [46]. Such experimental caveats notwithstanding, it is plausible to presume that a similar response mediated by microglia in the brain might occasionally occur in vivo in the presence of ORC. It would, however, likely be difficult to recapitulate a cystic mass in response to ORC, which can be considered a stochastic and idiosyncratic event, and careful consideration for the ethical use of animals for this purpose is paramount. Moreover, it is critical to further assess the impact of ORC on other cells of the CNS given its routine use in neurosurgical procedures. One might speculate that unknown additional inflammatory mechanisms could well be associated with the foreign body reactions. For instance, inflammasome activation and its downstream response to IL-1β production have been associated with an acute inflammatory response and cell death [47-49]. Although we have not fully explored the mechanistic understanding of acute or chronic inflammation associated with ORC in vivo, previous reports have shown the potential association between macrophage infiltration and foreign body reaction against ORC in mouse models [33]. Of course, neurosurgical procedures may often disrupt the blood–brain barrier [50] and therefore provide access to circulating monocytes in the peripheral blood, which in turn would further facilitate inflammatory cell infiltration and foreign body reaction alongside CNS-resident microglia.
In summary, these bedside-to-bench findings might provide a plausible initial mechanistic explanation for an immune ORC-mediated adverse effect that deserves more study. Histopathological analysis indicated foreign body reaction to residual ORC, including microglial activation, in the brain tumor resection cavity. Furthermore, in vitro experiments with microglial cells demonstrated that ORC affects cytokine production and cell viability. Given that ORC is conventionally used in neurosurgical procedures, we anticipate that our findings may be of clinical relevance and call for further mechanistic and comparative studies. As a matter of fact, Leisz et al. [32] have recently concluded that ORC may not be a suitable hemostatic agent in eloquent cortical areas of the CNS. We nonetheless do not recommend the prioritization of the avoidance of textiloma, which is relatively infrequent given its commonplace utilization, over sufficient postoperative hemostasis. The empiric and mechanistic observations reported here and elsewhere [32] rather serve to raise awareness of this complication of ORC and provide a possible explanation for this pathogenic phenomenon.
AUTHOR CONTRIBUTIONS
J.A.K., A.B.L., A.C., and P.K.A. managed the clinical care. C.M., F.H.F.T., A.B.L., D.I.S., R.P., and W.A. conceptualized the translational research. C.M., F.H.F.T., and A.B.L. performed the research. J.A.K., C.M., F.H.F.T., A.B.L., A.C., P.K.A., D.I.S., R.P., and W.A. analyzed the data. J.A.K., C.M., F.H.F.T., D.I.S., and W.A. wrote the initial draft of the manuscript, with subsequent editorial contributions from all other authors. J.A.K., D.I.S., R.P., and W.A. supervised the project. R.P. and W.A. funded the project. The order of co-first authors (J.A.K., C.M., and F.H.F.T.) was determined by alphabetical order of last names.
FUNDING INFORMATION
This work was supported by an award from the Levy-Longenbaugh Donor-Advised Fund (to R.P. and W.A.) and utilized shared resources supported by the Cancer Center Support Grant (P30CA072720) from the National Cancer Institute.
CONFLICT OF INTEREST STATEMENT
R.P. and W.A. are founders and equity shareholders of PhageNova Bio. R.P. is Chief Scientific Officer and a paid consultant of PhageNova Bio. R.P. and W.A. are founders and equity shareholders of MBrace Therapeutics; R.P. and W.A. serve as paid consultants for MBrace Therapeutics. R.P. and W.A. have Sponsored Research Agreements (SRAs) in place with PhageNova Bio and with MBrace Therapeutics. These arrangements are managed in accordance with the established institutional conflict-of-interest policies of Rutgers, The State University of New Jersey. This particular study falls outside of the scope of these SRAs.
ETHICS STATEMENT
Written informed consent was obtained for publication of the case. All patient data presented were de-identified.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




