Resource availability for CNS tumor diagnostics in the Asian Oceanian region: A survey by the Asian Oceanian Society of Neuropathology committee for Adapting Diagnostic Approaches for Practical Taxonomy in Resource-Restrained Regions (AOSNP-ADAPTR)
Chitra Sarkar and Shilpa Rao contributed equally to this manuscript.
Abstract
The shift toward a histo-molecular approach in World Health Organization classification of central nervous system tumors (WHO CNS5) emphasizes the critical role of molecular testing, such as next-generation sequencing (NGS) and DNA methylation profiling, for accurate diagnosis. However, implementing these advanced techniques is particularly challenging in resource-constrained countries. To address this, the Asian Oceanian Society of Neuropathology committee for Adapting Diagnostic Approaches for Practical Taxonomy in Resource-Restrained Regions (AOSNP-ADAPTR) was initiated to help pathologists in resource-limited regions to implement WHO CNS5 diagnoses using simpler diagnostic tools, mainly immunohistochemistry. An online survey by this group assessed the in-house/local availability of diagnostic resources and outsourcing capabilities across the Asian Oceanian region, covering 19 countries. Of 318 responding centers, the availability of molecular techniques in-house/locally was limited in lower middle-income countries (LMICs), with 29% having fluorescence in situ hybridization, 10.7% Sanger sequencing, and only 9.4% NGS. DNA methylation was largely unavailable in-house/locally in of LMICs, while in the whole region, its availability stood at a meager 4.4%. Though outsourcing for all diagnostic tests was an easily accessible alternative, outsourcing for NGS and DNA methylation was uncommon because of the financial burden on patients being a significant obstacle. The survey categorized centers into five resource levels (RLs) based on the in-house/local access to diagnostic techniques, which highlighted the variability and disparity in diagnostic capabilities across the region. High-income countries had 80% of centers with advanced RL IV, V resources, in contrast to LMICs, where 70.5% of centers fell into RL I–III. This comprehensive evaluation emphasizes the need for tailored resource level guidelines, with the aim of improving diagnostic accuracy and treatment as well as advocating better healthcare infrastructure in resource-constrained areas. However, the survey's reliance on responses from better-resourced centers may underestimate challenges in lower-resource settings, stressing the need for broader outreach and support.
1 INTRODUCTION
The fifth edition of the World Health Organization classification of central nervous system tumors (WHO CNS5) now integrates molecular alterations along with histopathology, emphasizing the crucial role of genetic testing for precise and accurate diagnoses [1]. Molecular characteristics identified through next-generation sequencing (NGS) and DNA methylation profiling have become essential diagnostic criteria for certain CNS tumors. This development poses significant challenges, particularly in economically disadvantaged countries, such as low-income and lower middle-income countries (LICs and LMICs). Limited human, infrastructural, and financial resources, coupled with a shortage of expertise, hinder the establishment of molecular genetic testing facilities in these regions.
Following the release of WHO CNS5 in December 2021, the Executive Committee of the Asian Oceanian Society of Neuropathology (AOSNP) recognized an urgent need to facilitate WHO diagnoses in settings lacking access to molecular testing. To address this, they launched the Asian Oceanian Society of Neuropathology committee for Adapting Diagnostic Approaches for Practical Taxonomy in Resource-Restrained Regions (AOSNP-ADAPTR)[2]. This initiative aims to provide a simplified approach for achieving diagnoses in line with WHO CNS5 using relatively basic diagnostic tools, particularly for pathologists in resource-limited regions. Notably, it does not suggest alternatives or modifications to the WHO classification but focuses on enabling its implementation under resource constraints.
The ADAPTR Steering Committee consists of AOSNP executives who lead neuro-oncological pathology practice in their respective countries/regions and are internationally recognized for their contributions to the field. ADAPTR also includes clinical and international advisory boards to ensure the clinical and global validity of its recommendations. Working Committee groups have been established, each led by a Chair and Co-Chair from the Steering Committee, along with four Working Committee members selected from the Asian Oceanian region based on their strong interest in neuro-oncological pathology (details of the committee members are provided in Supporting Information S1). The concept and outline of ADAPTR were presented during a session dedicated to this initiative on September 15, 2023, at the 20th International Congress of Neuropathology in Berlin, sponsored by the International Society of Neuropathology (ISN).
The Asian Oceanian region includes 48 countries across South, Southeast, East, Southwest, and Central Asia, along with 14 independent nations in Oceania (Figure 1; Table S1). With a population exceeding 4.7 billion in Asia and nearly 46 million in Oceania, this region accounts for 60% of the world's population. Socioeconomic diversity is significant, with countries classified by the World Bank (2023) into LIC, LMIC, upper middle-income (UMIC), and high-income (HIC) categories based on gross national income (GNI) (Table S2) [3]. This varied economic landscape introduces complexities in the accessibility and implementation of advanced diagnostic technologies for CNS tumors across the region.
Data on the availability of testing facilities for CNS tumors worldwide is limited, revealing significant disparities in access to molecular techniques. A single international survey conducted by Andreiuolo et al. [4], with the support of ISN in 2017, included participation from 314 centers across 48 countries, primarily in Europe and North America, with only 9 countries from the Asian Oceanian region. In this survey, immunohistochemistry (IHC) was widely available globally, but molecular techniques were unavailable in six countries (12.5%): Colombia, Croatia, Romania, United Arab Emirates (UAE), Uruguay, and Morocco. A recent survey in the United States indicated that advanced molecular testing of primary CNS tumors was limited even in academic hospitals with neurosurgery residency programs [5]. Only 40% of respondents reported that their institutions performed all 21 molecular alterations included in the survey. The survey also found that nearly 20% of gliomas still need to receive the diagnostic accuracy recommended by WHO CNS5. This indicates that the implementation of molecular profiling in routine clinical care needs to catch up to WHO CNS5 recommendations, even in HICs like the United States. However, the study's main limitation was the small sample size, with only 35 neuropathologists responding out of the 150 surveyed (30.4%).
Comprehensive data on the current status of in-house/local access to brain tumor diagnostic techniques in the Asian Oceanian region is lacking. Nevertheless, developing a pragmatic approach to diagnosing CNS tumors in this geographical area is crucial. Although not all molecular tests may be available in individual laboratories or centers, many can be accessed through outsourcing to central referral laboratories and commercial firms, wherever available and affordable. However, no data on the accessibility of outsourced resources in this region is available. The U.S. study reported that 14.3% of institutions sent all samples to outside facilities for molecular testing, while 77.1% used a combination of internal and external testing platforms [5].
- Documenting the in-house/local** availability of various resources* essential for diagnosing CNS tumors under the WHO CNS5 classification.
- Stratifying the Asian Oceanian region by country and economic status into distinct categories based on resource levels (RLs) to better understand the varying capacities and disparities in in-house/local resource availability for CNS tumor diagnosis.
A secondary objective was to assess how countries in this region with insufficient in-house/local resources manage CNS tumor diagnosis through outsourcing facilities. (*The term “resources” includes infrastructure, specialized machinery, equipment such as fluorescence microscopes, sequencers, high-throughput array scanners, etc., and consumable items like antibodies, probes, primers, etc., necessary for diagnostic tests. **“In-house/local” refers to the availability of/access to resources within the respective lab or Pathology/Neuropathology department of the treating institution/hospital.)
This survey thus aimed to serve as the groundwork for developing tailored recommendations according to resource levels applicable across all resource-restricted settings for major tumor groups, such as adult-type diffuse gliomas, pediatric-type gliomas, embryonal tumors, and others.
2 MATERIALS AND METHODS
The ADAPTR Steering Committee members conceptualized an online survey, for which first-line and second-line questionnaires were formulated using Google® forms and refined through consensus among the members (Supporting Information S1). The first-line questionnaire addressed the in-house/local availability of resources within centers, while the second-line questionnaire focused on outsourcing facilities in centers lacking adequate in-house/local resources.
A targeted approach was undertaken to ensure the effective dissemination of the online survey across the Asian Oceanian region. Efforts were directed at establishing contact with as many countries as possible within this region. Out of the 48 countries listed (Table S1), 19 could be successfully reached, including 4 in East Asia, 5 in South Asia, 6 in Southeast Asia, 2 in Western Asia, and 2 in Oceania. This outreach was primarily facilitated through the support of current AOSNP members and their personal networks as well as through references to publications in peer-reviewed journals. However, countries in Central Asia and most in Western Asia (except Turkey and Jordan) and Oceania (except Australia and New Zealand) could not be contacted, as they are not represented in the current AOSNP, and no current members had personal networks in these regions. Additionally, no registered official neuropathology societies from these regions are listed on the ISN website (https://www.intsocneuropathol.com) or featured in the society news section of Brain Pathology, the official journal of the ISN.
Next, a designated contact person was identified in each of the 19 countries as one who was engaged in the active practice of neuro-oncological pathology (list provided in the acknowledgement and details in Supporting Information S1). Each such designated contact thus identified was then entrusted with the task of distributing the Google® survey form. The contact person shared this form through national pathology/neuropathology societies among their members and personally sent it to pathologists and neuropathologists in that country. The emphasis was on establishing a personal connection with professionals familiar with pathology centers practicing neuro-oncological pathology in their respective countries. This multi-pronged approach, utilizing both formal society channels and individual outreach, ensured a targeted outreach of the survey form within the neuropathology community.
Each diagnostic unit (university/institute/laboratory/hospital) that responded to the questionnaire was referred to as “respondent center” (hitherto mainly referred to as only “center” in the text for the sake of brevity, convenience, and avoiding repetition). The responses from various respondent centers within each country were then organized into spreadsheets and forwarded to the respective contact person for a validation check. Contact persons were instructed that the data should specifically pertain to the status of individual pathology units, with the directive that each center contributes only one response. Any identified errors, discrepancies, or instances of more than one respondent from a single center were diligently rectified throughout the validation process. This review and correction process played a pivotal role in enhancing the reliability of the collected data. Because of the unavailability of access to Google® forms in China, responses from this country were obtained through direct email submissions, facilitated by the generous assistance of Dr. Yue-Shan Piao (Department of Neuropathology, Xuanwu Hospital, Capital Medical University, Beijing, China).
Since the survey questions in the first-line questionnaire focused on in-house/local access to resources, maximum care was taken by requesting all the designated contacts to personally clarify from centers in case they had any doubt regarding this.
- Number and percentage out of total responses received (combined LMIC, UMIC, and HIC).
- Number and percentage out of total responses received separately from LMIC, UMIC, and HIC (analysis by socioeconomic status as per World Bank criteria).
- Number and percentage out of total responses received country-wise (analysis on a country-by-country basis).
The results presented in this report exclusively represent responses only from centers in each country that participated in the survey. Unfortunately, we do not have any data about the number of non-responders (those who received the survey form but did not respond), which is one of the limitations of this study.
A second-line questionnaire (Supporting Information S1) was circulated to only the nodal/contact persons in each of the 19 countries as they were actively practicing neuro-oncological pathology in their respective countries and were well-updated regarding the available facilities.
The questionnaire first addressed the issue of the availability of outsourcing facilities in each of the 19 countries when in-house/local resources were lacking. Subsequently, the focus was to ascertain the common tests outsourced from each country (e.g., IHC, fluorescence in situ hybridization (FISH), Sanger sequencing, MGMT promoter methylation, NGS, and DNA methylation profiling) and the frequency of outsourcing, particularly for NGS and DNA methylation. The survey also sought to determine the destination of outsourcing (e.g., higher resource level referral laboratories in academic institutions/commercial firms/private labs within or outside the country) and the availability of central referral labs within each country. Additional questions explored who primarily decides to request outsourcing and who covers the cost of testing.
3 RESULTS
3.1 Responses to the first line questionnaire: in-house/local availability of resources for diagnosis
3.1.1 Overall responses
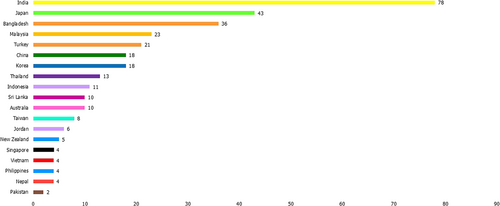
A total of 19 countries could be contacted and responded to the survey (Figure 2), including 8 LMICs (42%), 5 UMICs (26%), and 6 HICs (32%) (Table 1; Figures 2-5). Afghanistan was the only LIC in this region, but we could not identify any pathologists who provided brain tumor diagnostics in this country. From these 19 countries, we received 318 responses, with the highest number coming from India (n = 78), followed by Japan (n = 43). Most responses were from LMICs, accounting for 46.9% (n = 149). UMIC responses comprised 25.5% (n = 81), and HIC responses constituted 27.7% (n = 88).
| Variable | Total (n = 318; %) | High income countries 88 (27.7) | Upper-middle income countries 81 (25.5) | Low-middle income countries 149 (46.8) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | AUS (n = 10) | JPN (n = 43) | NZL (n = 5) | PRK (n = 18) | SGP (n = 4) | TWN (n = 8) | CHN (n = 18) | JOR (n = 6) | MYS (n = 23) | THA (n = 13) | TUR (n = 21) | BGD (n = 36) | IDN (n = 11) | IND (n-78) | LKA (n = 10) | NPL (n = 4) | PAK (n = 2) | PHL (n = 4) | VNM (n = 4) | |
| Hospital type (n, %) | Public | 8 (80) | 9 (20.9) | 4 (80) | 1 (5.6) | 2 (50) | 4 (50) | 12 (67) | 2 (33.3) | 12 (52.2) | 8 (62) | 5 (23.8) | 0 | 8 (73) | 9 (11.5) | 8 (80) | 1 (25) | 0 | 0 | 3 (75) |
| Private | 2 (20) | 0 | 1 (20) | 0 | 0 | 1 (12.5) | 0 | 2 (33.3) | 5 (21.7) | 0 | 3 (14.3) | 20 (55.6) | 2 (18) | 31 (39.8) | 1 (10) | 2 (50) | 1 (50) | 3 (75) | 0 | |
| Institute | 0 | 1 (2.3) | 0 | 2 (11.1) | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | 31 (39.8) | 0 | 0 | 0 | 0 | 0 | |
| Uni | 0 | 33 (76.8) | 0 | 15 (83.3) | 2 (50) | 3 (37.5) | 6 (33) | 1 (16.7) | 6 (26.1) | 5 (38) | 13 (61.9) | 16 (44.4) | 1 (9) | 7 (8.9) | 1 (10) | 1 (25) | 1 (50) | 1 (25) | 1 (25) | |
| Number of CNS tumors diagnosed per year (n, %) | ≤50 | 1 (10) | 12 (27.9) | 0 | 6 (33.4) | 2 (50) | 2 (25) | 0 | 3 (50) | 9 (39.1) | 6 (46) | 0 | 24 (66.7) | 7 (64) | 6 (7.7) | 4 (40) | 1 (25) | 0 | 1 (25) | 1 (25) |
| 51–250 | 4 (40) | 27 (62.8) | 4 (80) | 4 (22.2) | 1 (25) | 5 (62.5) | 2 (11) | 3 (50) | 12 (52.2) | 3 (23) | 12 (57.1) | 4 (11.1) | 3 (27) | 25 (32.1) | 2 (20) | 3 (75) | 0 | 3 (75) | 0 | |
| 251–500 | 3 (30) | 4 (9.3) | 1 (20) | 2 (11.1) | 1 (25) | 1 (12.5) | 2 (11) | 0 | 2 (8.7) | 2 (15) | 6 (28.6) | 4 (11.1) | 1 (9) | 21 (26.9) | 3 (30) | 0 | 0 | 0 | 2 (50) | |
| 501–1000 | 2 (20) | 0 | 0 | 4 (22.2) | 0 | 0 | 4 (22) | 0 | 0 | 1 (8) | 3 (14.3) | 3 (8.3) | 0 | 15 (19.2) | 0 | 0 | 2 (100) | 0 | 0 | |
| >1000 | 0 | 0 | 0 | 2 (11.1) | 0 | 0 | 10 (56) | 0 | 0 | 1 (8) | 0 | 1 (2.8) | 0 | 11 (14.1) | 1 (10) | 0 | 0 | 0 | 1 (25) | |
| WHO CNS5 (2021) integrated diagnosis (n, %) | Yes | 9 (90) | 25 (58.1) | 4 (80) | 11 (61.1) | 4 (100) | 4 (50) | 13 (72) | 1 (16.7) | 12 (52.2) | 8 (62) | 12 (57.1) | 2 (5.6) | 5 (45.4) | 51 (65.4) | 7 (70) | 3 (75) | 2 (100) | 0 | 3 (75) |
| No | 1 (10) | 4 (9.3) | 0 | 2 (11.1) | 0 | 1 (12.5) | 1 (6) | 2 (33.3) | 2 (8.7) | 1 (8) | 9 (42.9) | 34 (94.4) | 1 (9.2) | 5 (6.4) | 1 (10) | 0 | 0 | 3 (75) | 0 | |
| In some cases | 0 | 14 (32.6) | 1 (20) | 5 (27.8) | 0 | 3 (37.5) | 4 (22) | 3 (50) | 9 (39.1) | 4 (31) | 0 | 0 | 5 (45.4) | 22 (28.2) | 2 (20) | 1 (25) | 0 | 1 (25) | 1 (25) | |
| Access to radiological data (MRI with contrast) (n, %) | Yes | 10 (100) | 40 (93.1) | 5 (100) | 15 (83.3) | 4 (100) | 8 (100) | 16 (88.9) | 6 (100) | 16 (69.6) | 8 (61.5) | 16 (76.2) | 20 (55.6) | 7 (63.6) | 59 (75.6) | 8 (80) | 3 (75) | 2 (100) | 3 (75) | 3 (75) |
| No | 0 | 0 | 0 | 1 (5.6) | 0 | 0 | 0 | 0 | 5 (21.7) | 0 | 0 | 0 | 1 (9.1) | 3 (3.9) | 1 (10) | 0 | 0 | 0 | 0 | |
| In some cases | 0 | 3 (6.9) | 0 | 2 (11.1) | 0 | 0 | 2 (11.1) | 0 | 2 (8.7) | 5 (38.5) | 5 (23.8) | 16 (44.4) | 3 (27.3) | 16 (20.5) | 1 (10) | 1 (25) | 0 | 1 (25) | 1 (25) | |
| In-house access to routine IHC (n, %) | Yes | 9 (90) | 42 (97.7) | 5 (100) | 18 (100) | 4 (100) | 8 (100) | 17 (94) | 5 (83.3) | 17 (73.9) | 9 (69) | 20 (95.2) | 5 (13.9) | 3 (27) | 60 (76.9) | 8 (80) | 0 | 2 (100) | 4 (100) | 4 (100) |
| Few Abs | 1 (10) | 1 (2.3) | 0 | 0 | 0 | 0 | 0 | 1 (16.7) | 5 (21.7) | 3 (23) | 1 (4.8) | 6 (16.7) | 1 (9) | 10 (12.8) | 2 (20) | 1 (25) | 0 | 0 | 0 | |
| No | 0 | 0 | 0 | 0 | 0 | 0 | 1 (6) | 0 | 1 (4.4) | 1 (8) | 0 | 25 (69.4) | 7 (64) | 8 (10.3) | 0 | 3 (75) | 0 | 0 | 0 | |
| In-house access to surrogate IHC molecular markers (n, %) | Yes | 8 (80) | 33 (76.7) | 1 (20) | 13 (72.2) | 2 (50) | 7 (87.5) | 13 (72) | 1 (16.7) | 3 (13.1) | 5 (38) | 14 (66.7) | 5 (13.9) | 0 | 25 (32.1) | 0 | 0 | 1 (50) | 0 | 3 (75) |
| Few Abs | 1 (10) | 2 (4.7) | 4 (80) | 5 (27.8) | 2 (50) | 1 (12.5) | 4 (22) | 3 (50) | 15 (65.2) | 4 (31) | 6 (28.5) | 1 (2.8) | 2 (18) | 36 (46.1) | 6 (60) | 1 (25) | 1 (50) | 4 (100) | 0 | |
| No | 1 (10) | 8 (18.6) | 0 | 0 | 0 | 0 | 1 (6) | 2 (33.3) | 5 (21.7) | 4 (31) | 1 (4.8) | 30 (83.3) | 9 (82) | 17 (21.8) | 4 (40) | 3 (75) | 0 | 0 | 1 (25) | |
| In-house access to FISH technique (n, %) | Yes | 9 (90) | 23 (53.5) | 2 (40) | 14 (77.8) | 3 (75) | 6 (75) | 13 (72) | 1 (16.7) | 7 (30.4) | 3 (23) | 12 (57.1) | 3 (8.3) | 0 | 35 (44.9) | 2 (20) | 0 | 1 (50) | 1 (25) | 1 (25) |
| No | 1 (10) | 20 (46.5) | 3 (60) | 4 (22.2) | 1 (25) | 2 (25) | 5 (28) | 5 (83.3) | 16 (69.6) | 10 (77) | 9 (42.9) | 33 (91.7) | 11 (100) | 43 (55.1) | 8 (80) | 4 (100) | 1 (50) | 3 (75) | 3 (75) | |
| In-house access to Sanger sequencing (n, %) | Yes | 2 (20) | 24 (55.8) | 1 (20) | 12 (66.7) | 3 (75) | 4 (50) | 12 (67) | 1 (16.7) | 2 (8.7) | 1 (8) | 2 (9.5) | 0 | 0 | 16 (20.5) | 0 | 0 | 0 | 0 | 0 |
| No | 8 (80) | 19 (44.2) | 4 (80) | 6 (33.3) | 1 (25) | 4 (50) | 6 (33) | 5 (83.3) | 21 (91.3) | 12 (92) | 19 (90.5) | 36 (100) | 11 (100) | 62 (79.5) | 10 (100) | 4 (100) | 2 (100) | 4 (100) | 4 (100) | |
| In-house access to NGS (n, %) | Yes | 5 (50) | 8 (18.6) | 0 | 18 (100) | 3 (75) | 2 (25) | 8 (44) | 1 (16.7) | 4 (17.4) | 1 (8) | 5 (23.8) | 0 | 0 | 13 (16.7) | 0 | 0 | 1 (50) | 0 | 0 |
| No | 5 (50) | 35 (81.4) | 5 (100) | 0 | 1 (25) | 6 (75) | 10 (56) | 5 (83.3) | 19 (82.6) | 12 (92) | 16 (76.2) | 36 (100) | 11 (100) | 65 (83.3) | 10 (100) | 4 (100) | 1 (50) | 4 (100) | 4 (100) | |
| In-house access to DNA methylation profiling (n, %) | Yes | 2 (20) | 2 (4.6) | 1 (20) | 6 (33.3) | 0 | 0 | 2 (11) | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No | 8 (80) | 41 (95.4) | 4 (80) | 12 (66.7) | 4 (100) | 8 (100) | 16 (89) | 5 (83.3) | 23 (100) | 13 (100) | 21 (100) | 36 (100) | 11 (100) | 78 (100) | 10 (100) | 4 (100) | 2 (100) | 4 (100) | 4 (100) | |
| Resource level (RL) (n, %) | RL I | 0 | 1 (2.3) | 0 | 0 | 0 | 0 | 1 (6) | 0 | 1 (4.3) | 0 | 0 | 25 (69.4) | 7 (63.6) | 7 (8.9) | 0 | 3 (75) | 0 | 0 | 0 |
| RL II | 0 | 1 (2.3) | 0 | 0 | 0 | 0 | 0 | 2 (33.3) | 4 (17.4) | 4 (31) | 0 | 4 (11.1) | 2 (18.2) | 9 (11.5) | 4 (40) | 0 | 0 | 0 | 1 (25) | |
| RL III | 0 | 10 (23.3) | 3 (60) | 0 | 1 (25) | 2 (25) | 3 (17) | 3 (50) | 11 (47.8) | 6 (46) | 9 (42.9) | 4 (11.1) | 2 (18.2) | 27 (34.6) | 4 (40) | 1 (25) | 0 | 3 (75) | 2 (50) | |
| RL IV | 5 (50) | 22 (51.2) | 1 (20) | 0 | 0 | 4 (50) | 6 (33) | 0 | 3 (13.1) | 2 (15) | 7 (33.3) | 3 (8.3) | 0 | 22 (28.3) | 2 (20) | 0 | 1 (50) | 1 (25) | 1 (25) | |
| RL V | 5 (50) | 9 (20.9) | 1 (20) | 18 (100) | 3 (75) | 2 (25) | 8 (44) | 1 (16.7) | 4 (17.4) | 1 (8) | 5 (23.8) | 0 | 0 | 13 (16.7) | 0 | 0 | 1 (50) | 0 | 0 | |
- Note: Country codes as per the ISO 3166-1 alpha-3 codes (in alphabetical order): AUS; Australia, BGD; Bangladesh, CHN; China, IDN, Indonesia, IND; India, JOR; Jordan, JPN; Japan, LKA; Sri Lanka, MYS; Malaysia, NPL; Nepal, NZL; New Zealand, PAK; Pakistan, PHL; Philippines, PRK; Korea, SGP; Singapore, THA; Thailand, TUR; Türkiye, TWN; Taiwan, VNM; Vietnam. Color-code: green: LMIC; orange: UMIC; black: HIC.
- Abbreviations: Abs, antibodies; HIC, high-income; IHC, immunohistochemistry; LMIC, lower middle-income; NGS, next-generation sequencing; UMIC, upper middle-income; Uni; University/Institute; WHO CNS5, World Health Organization classification of Central Nervous System tumors.
3.1.2 Analysis of the first-line survey questions
Type of hospital
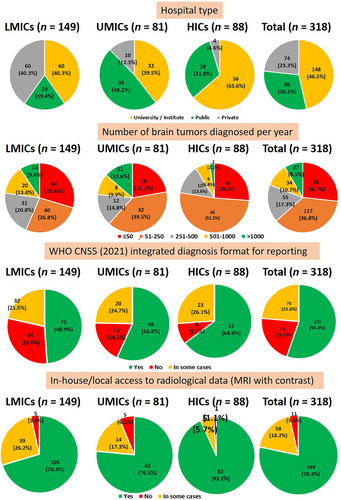
Most centers that responded were universities or institutes, making up 46.5% of the total (Table 1; Figures 3-5). This trend was consistent across different income levels, with 40.3% in LMICs, 39.5% in UMICs, and the highest percentage in HICs at 63.6%. Public sector hospitals represented 30.2% of the responses, with the highest occurrence in UMICs (48.2%) and the lowest in LMICs (19.4%). Private hospitals and laboratories accounted for 23.3% of the responses, most prevalent in LMICs (40.3%) and least in HICs (4.6%).
Number of brain tumor cases diagnosed per year
Approximately a quarter (26.7%) of respondent centers diagnosed 50 or fewer cases yearly. Most centers (36.8%) were involved in diagnosing between 51 and 250 brain tumor cases annually. This was particularly notable in UMICs (39.5%) and HICs (51.2%). An observation was that 22.8% of centers in LMICs and 23.5% in UMICs diagnosed more than 500 brain tumor cases annually, in contrast to only 9% in HICs. Overall, 19.2% of centers in this region reported diagnosing over 500 cases per year.
Adaptation of the WHO CNS5 integrated diagnosis format for reporting
The predominant approach among respondent centers was utilizing the WHO classification for reporting pathological diagnoses. Specifically, 55.3% of centers used it in all cases and 21.5% in some cases, totaling 76.8% overall. Adoption of the WHO CNS5 classification was most prominent in HICs (64.8%) and least in LMICs (48.9%). In UMICs, 56.8% of centers employed this classification for reporting.
Availability of radiological data (mainly magnetic resonance imaging)
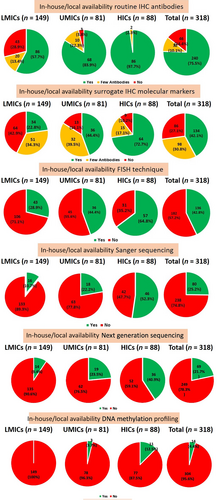
Most respondent centers had in-house/local access to radiological data in all cases (78.3%) or in some cases (18.2%). This trend was consistent across LMICs (70.4% and 26.2%), UMICs (76.5% and 17.3%), and HICs (93.2% and 5.7%). As a result, magnetic resonance imaging (MRI) was unavailable in only 3.5% of the centers overall: 3.4% in LMICs, 6.2% in UMICs, and 1.1% in HICs.
Availability of basic diagnostic IHC markers
Most respondent centers (75.5%) in this region had access to routine, basic diagnostic IHC antibodies. However, 14.4% of centers lacked in-house/local access, and 10% had only a few antibodies available. The lack of routine IHC antibodies was most pronounced in LMICs (28.9%), with the highest rates of non-availability being in centers in Nepal (100%), Bangladesh (86%), and Indonesia (73%).
Availability of surrogate IHC molecular markers
A total of 42.1% of all respondent centers reported having in-house/local access to advanced IHC markers, including surrogate molecular markers. About 30.8% of centers had only a few of these markers available, while 27.1% did not use any advanced IHC markers.
When analyzing the distribution by country, LMICs showed the least utilization, with 34.3% of centers using a limited set of antibodies and 42.9% not employing any (totaling 77%). Notably, all centers (100%) in Indonesia, Nepal, the Philippines, and Sri Lanka either did not have these surrogate IHC markers or used only a few. In Bangladesh, this percentage was 86%; in India, it stood at 68%.
The availability of surrogate antibodies within UMICs varied. For instance, Malaysia, Jordan, and Thailand lacked or had few surrogate antibodies in 87%, 83%, and 62% of their centers. Conversely, China and Turkey reported the availability of surrogate markers in 70% of their centers.
In HICs, approximately 80% or more of the centers in countries like Australia, Japan, Korea, and Taiwan had in-house/local access to surrogate markers. The only exception was New Zealand, where these markers were available in only 20% of responding centers.
Availability of FISH
Regarding in-house/local FISH accessibility, 42.8% of respondent centers had access overall. In LMICs, availability was lower at 29%. Some countries within this category, such as Indonesia and Nepal, reported a complete absence of in-house/local access to FISH. Bangladesh had FISH available in only 8% of centers, while access to FISH in the centers in Philippines, Vietnam, and Sri Lanka ranged from 20% to 25%. India reported the highest in-house/local access among LMICs at 45%.
Among UMICs, the overall in-house/local access rate was 44.4%, significantly varying among nations. Thailand, Malaysia, and Jordan reported that FISH was unavailable in 70% to 83% of their centers. In contrast, China and Turkey exhibited higher availability rates, at 72% and 60%, respectively.
In HICs, 64.8% of centers had in-house/local access to FISH. Availability varied from 40% to 90%, with Australia having the highest accessibility rate at 90%. New Zealand and Japan were at the lower end of the spectrum, with 40% and 53.5%, respectively.
Availability of Sanger sequencing
A significant finding was that 74.8% of respondent centers in this region lacked Sanger sequencing facilities. Overall, only 25.2% of centers reported having in-house/local access, with the lowest availability observed in LMICs at 10.7%, followed by UMICs at 22.2% and HICs at 52.3%. Several countries in LMICs, including Bangladesh, Nepal, Indonesia, the Philippines, Sri Lanka, Vietnam, and Pakistan, reported a complete absence of in-house/local access to Sanger sequencing in their centers. In India, only 20% of centers had access to this facility. Similarly, in UMICs such as Thailand, Malaysia, Jordan, and Turkey, 83% to 92% of centers lacked in-house/local access to Sanger sequencing. China had the highest availability at 67%. In HICs, in-house/local access was highest in centers in Korea (75%), followed by Japan (56%).
Availability of NGS
The data on in-house/local access to NGS revealed substantial disparities among respondent centers across different regions, with only 21.7% having access overall. Specifically, availability was notably low at 9.4% in LMICs, 23.5% in UMICs, and comparatively higher at 40.9% in HICs. In LMICs, centers in countries like Bangladesh, Indonesia, Nepal, the Philippines, Sri Lanka, and Vietnam completely lacked in-house/local access to NGS. Only 17% of centers in India reported having this capability.
Within UMICs, a significant majority—75%–92%—lacked in-house/local NGS access, except for China, where 56% of centers reported availability. Among HICs, NGS was available in most centers in Korea that responded to the survey. In Australia, only 50% of centers had in-house/local access. Japan and Taiwan exhibited much lower in-house/local availability at 18.6% and 25% of centers, respectively. Interestingly, there was no in-house/local NGS access among respondents from New Zealand.
Availability of DNA methylation profiling
The overall in-house/local accessibility of DNA methylation profiling across the Asian Oceanian region was notably limited, at a mere 4.4% of the respondent centers. It is worth mentioning that no laboratories in LMICs offered DNA methylation profiling services, despite around 22% of their centers managing over 500 brain tumor cases annually.
In UMICs, in-house/local access for diagnostic purposes was absent in centers in Thailand, Turkey, and Malaysia. Only China and Jordan reported having centers with this capability. As a result, among UMICs, DNA methylation profiling was available in only 3.7% of respondent centers.
HICs showed higher availability, with centers in Australia, Korea, Japan, and New Zealand offering in-house/local access. However, even in HICs, the overall in-house/local availability of DNA methylation profiling remained low at 12.5%.
3.2 Analysis of resource levels based on in-house/local availability of resources
Survey findings have outlined a varied spectrum of in-house/local resource availability, leading to the classification of RLs from I to V (Figure 5) [2].
3.2.1 Resource level I (RL I)
This level entails diagnostic evaluation solely through histopathology using H&E staining, optionally augmented by specialized histochemical stains such as reticulin and periodic acid-Schiff.
3.2.2 Resource level II (RL II)
At RL II, basic IHC markers, including GFAP, synaptophysin, OLIG2, vimentin, MIB-1, EMA, CD34, and others, are accessible.
3.2.3 Resource level III (RL III)
At RL III, specific or surrogate IHC markers are available for significant molecular events, such as IDH1R132H, ATRX, H3K27M, H3K27me3, INI1, p53, L1CAM, BRAFV600E, beta-catenin, GAB1, YAP1, and others.
3.2.4 Resource level IV (RL IV)
At this RL, there is the capacity for fundamental molecular techniques, namely FISH (for detecting copy number alterations like 1p/19q codeletion, EGFR, MYC, and MYCN amplification, as well as PTEN and CDKN2A/B deletions) and/or Sanger sequencing (for analyzing single-gene variants in genes such as IDH1, IDH2, BRAF, and the TERT promoter, among others).
3.2.5 Resource level V (RL V)
Advanced molecular techniques such as NGS and/or DNA methylation arrays are included in this RL.
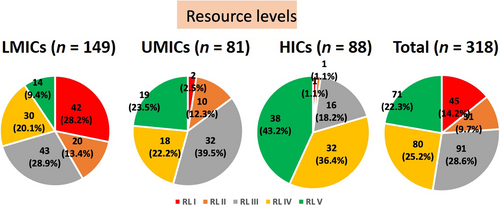
Our survey highlighted distinct patterns in RLs across countries. In LMICs, 70.5% of respondent centers fell into RL I to III categories (RL I 28.2%, RL II 13.4%, and RL III 28.9%), contrasting sharply with HICs, where these levels accounted for only 20.4% (RL I 1.1%, RL II 1.1%, and RL III 18.2%). Particularly in LMICs like Bangladesh, Indonesia, and Nepal, a significant proportion of respondent centers belonged to RL I, ranging from 63% to 75%. In UMICs, RL III centers were more prevalent, comprising nearly 40%. RL IV and V centers were more prevalent in HICs, constituting 36.4% and 43.2%, respectively (totaling 79.6%). In contrast, in LMICs and UMICs, RL IV and V centers together accounted for only 29.5% and 45.8%, respectively. In summary, LMICs have fewer RL IV and V centers than HICs, where most centers are categorized under RL IV and V.
3.3 Responses to the second-line questionnaire: availability of facility for outsourcing of tests when in-house/local access is not available
Responses to the second-line questionnaire were received from representatives in 15 of the 19 countries (excluding Taiwan, Philippines, Singapore, and Turkey) (Table S3). It revealed that outsourcing facilities were accessible across LMICs, UMICs, and HICs for all types of tests, including IHC, FISH, Sanger sequencing, MGMT promoter methylation, NGS, and DNA methylation profiling. Samples were outsourced both to specialized referral labs in academic institutions/hospitals as well as to commercial firms or private labs within the country or abroad.
The most frequently outsourced tests from centers in these countries included IHC for surrogate markers, FISH, Sanger sequencing, and MGMT promoter methylation. Notably, even basic IHC markers were outsourced from some LMICs like Nepal, Sri Lanka, Indonesia, and Vietnam. Outsourcing for NGS varied from “sometimes to rarely” across LMICs, UMICs, and HICs (7 Sometimes, 6 Rarely, 1 Never, 1 Often/out of 15). However, outsourcing for DNA methylation profiling was mainly “rare to never” (9 Rarely, 2 Never, 3 Sometimes, 1 Often/out of 15) across all income levels (Table S3).
In many LMICs, such as Bangladesh, Nepal, Indonesia, Sri Lanka, and Vietnam, most outsourcing for all tests was directed to commercial firms or private labs, whether within the country or abroad. In contrast, in UMICs and HICs, specialized referral labs were typically available in institutes or hospitals within the country. However, there was no mandatory requirement or standard protocol for referral, leading some centers in UMICs and HICs to also rely on commercial firms or private labs for outsourcing tests. Another strategy adopted by centers in LMICs and UMICs lacking advanced diagnostic facilities was to participate in twinning programs with highly specialized referral labs outside their countries, often in HICs. For instance, Aga Khan University Hospital in Karachi, Pakistan (AKUH), and King Hussein Cancer Center in Jordan were part of a twinning program with SickKids in Toronto, Canada.
A notable finding across all 15 countries in the study was that molecular testing outsourcing was primarily initiated by clinicians (neurosurgeons and oncologists), with additional requests from pathologists or patients (Table S3). Moreover, in LMICs, UMICs, and HICs, patients predominantly bore the cost of outsourcing for molecular testing (13/15). In some countries, patient insurance and/or the referring department also contribute to covering these costs. Only two respondents (from Australia and Korea) indicated that the government also contributed to the cost. Only a few respondents mentioned that their associated institutions covered the costs, especially when tests were part of a twinning program or conducted for research purposes. Therefore, the high financial burden was the primary obstacle for patients regarding outsourcing molecular testing, especially for NGS and DNA methylation profiling.
4 DISCUSSION
This is possibly the most extensive study evaluating the in-house/local availability and access by outsourcing of various diagnostic techniques for CNS tumors according to the WHO CNS5 classification in the Asian Oceanian region. This region, with diverse socioeconomic conditions, faces significant disparities in accessing advanced diagnostics, leading to considerable variability in the level of diagnosis, particularly for CNS tumors requiring molecular testing. The analysis includes a country-wise breakdown of responses, categorized by income levels (LMICs, UMICs, and HICs) and RLs (RL I to RL V), adding valuable context to the survey results. Thus, this survey is an effort to provide a comprehensive understanding of the current status of brain tumor diagnostics in the Asian Oceanian region and offer detailed insights into regional variations and disparities.
The distribution analysis of centers (public, private, institutional, and university) and the annual number of brain tumor cases diagnosed revealed crucial insights. Notably, a significant percentage of respondent centers in LMICs (22.8%) and UMICs (23.5%) diagnose over 500 cases annually, which may reflect the larger populations of these countries. However, it highlights the need for infrastructure development in these regions to meet growing diagnostic demands. The predominance of private hospitals and laboratories in LMICs contrasts with the prevalence of public hospitals and universities/institutes in UMICs, indicating a substantial financial burden on patients in LMICs and highlighting the importance of addressing the economic aspects of diagnostic services. The dominance of private centers in LMICs is presumed to be caused by the better financial status of these centers compared to the poor funding and infrastructure in government-run institutes and universities. However, data to support this was not collected in the survey.
The adoption of the WHO CNS5 classification and the integrated diagnosis format was most prevalent in respondent centers of HICs at 64.8%, with the lowest adherence observed in LMICs, reiterating the better in-house/local access to sophisticated diagnostic techniques in HICs as compared to UMICs and LMICs. Further, it is also important to point out that most centers in this region are not dedicated neuropathology centers. They are pathology departments or divisions (with or without trained neuropathologists) that report neuropathology as part of the general pathology service. Consequently, many centers need more expertise to provide integrated diagnosis as per WHO CNS5 guidelines.
The survey also revealed that radiological data was generally well available across respondent centers in every country (78.3%). In contrast, nearly 30% of centers in LMIC lacked basic IHC antibodies, and many LMIC and UMIC centers had limited or no surrogate IHC markers (77% and 56%, respectively). This indicates heavy reliance on basic staining methods like H&E without IHC supplementation for diagnosing CNS tumors in many LMICs. This is why ADAPTR has proposed to incorporate radiological data to improve the accuracy of the final pathological diagnosis in its guidelines.
Regarding the availability of molecular tests, such as FISH, Sanger sequencing, NGS, and DNA methylation profiling, the survey revealed constraints and disparities that could impact the quality of diagnostic services, particularly for tumors like oligodendrogliomas (requiring analysis of the 1p/19q status), IDH-wildtype glioblastomas (Sanger sequencing), high-grade astrocytoma with piloid features (DNA methylation), and some intracranial sarcomas (NGS). The data indicates that FISH was more commonly available in the Asian Oceanian region (42.8%), while Sanger sequencing was limited to only 25% of respondent centers. In-house/local availability of FISH in HICs ranged from 40% to 90%. It is possible that some centers may not have FISH available anymore because they use other, more advanced tests to find the alterations that were previously identified using FISH.
NGS was available in approximately 21% of the total respondent centers, likely because of its widespread use in general pathology departments for systemic cancers like lung and breast. However, caution is warranted because standard NGS cancer panels used in these departments often do not adequately cover specific markers for CNS tumors, necessitating additional tests such as FISH and Sanger sequencing, thereby increasing diagnostic costs. It is also noteworthy that the government healthcare/medical insurance system does not currently cover NGS for diagnostic purposes financially in some countries like Japan and China.
DNA methylation profiling was locally accessible in as few as 4.4% of the total respondent centers in this region, including only 12.5% in HICs. In-house/local DNA methylation profiling is only accessible in select countries within the region: Jordan, China, Australia, New Zealand, Korea, and Japan. Among these, governmental medical coverage approves diagnostic use solely in Jordan, Australia, and New Zealand, while in China, Korea, and Japan, the test is not covered currently under national healthcare insurance, rendering this a costly test. Therefore, though locally accessible, this test is done in these countries mainly for research purposes, and sometimes pathologists incorporate the results in their reports to provide integrated diagnosis, especially in diagnostically challenging cases.
The survey results were used to classify countries into different RLs, aiming to provide tailored guidance that acknowledges and addresses the diversity of available resources. The findings showed that a higher percentage of respondent centers in LMICs and UMICs fall into RLs I–III (70.5% and 54.3%, respectively). In contrast, RL IV and V centers were more prevalent in HICs, constituting about 80% overall. This highlights the necessity for recommendations primarily based on histology and IHC, supported by radiology, in these settings. In a retrospective analysis by Koy et al. on the impact of the WHO CNS5 classification system in low-resource settings, the authors emphasized the practical feasibility and economic viability of relying on simpler techniques rather than complex molecular methods in LMICs and UMICs [6]. Our categorization of centers according to RLs is distinctive as it explains disparities between countries and within countries. It allows each center to be classified based on its specific resource level and follow guidelines accordingly rather than applying universal guidelines. Regarding country designations, terms like “low and lower middle-income countries” are well-established. Yet, categorizing countries solely by income levels may not adequately capture the variations and diversity within each country. Our approach of categorizing centers within countries by resource level is likely more helpful than subjective income-based designations, especially given the substantial disparities even within HIC countries.
While our survey primarily focused on in-house/local facilities, it also assessed the availability of outsourcing in this region. Through outsourcing, access to all diagnostic techniques was available across all countries in this region (LMICs, UMICs, and HICs). In LMICs, outsourcing was predominantly to commercial firms/private labs either within the country or abroad, mainly because of the lack of specialized referral academic labs, except for a few with limited facilities. Conversely, UMICs and HICs often utilized a combination of academic labs and commercial labs for outsourcing. Our survey results on outsourcing facilities in the Asia Oceanian region align closely with a study conducted in centers with academic residency programs in the United States by Parker et al. [5]. They found that 14.3% of institutions exclusively relied on external facilities for testing, with 77.1% using a mix of internal and external methods. Notably, DNA methylation profiling was outsourced mainly (75%). External testing was commonly conducted at other academic hospital reference laboratories (40%) and commercial laboratories (51.4%).
Similar to our survey, Parker et al. also noted that testing was typically initiated by neurosurgeons (54.3%), oncologists (77.1%), or pathologists (68.6%), with patients initiating testing in only 20% of cases [5]. However, a drawback of this referral process observed in our survey was the lack of feedback to pathologists unless they actively sought communication with requesting clinicians. This communication gap hindered pathologists' involvement in interpreting test results, potentially complicating diagnostic decisions, especially in LMICs where adherence to advanced molecular guidelines may not always alter treatment decisions significantly. Additionally, Parker et al. highlighted that outsourcing often involves longer turnaround times than in-house/local testing, making it less effective in clinical decisions [5].
According to our survey results, patients predominantly bear the cost of these outsourced tests. In addition, the patient insurance/referring department/government also contributes to covering these costs, whose policy varies tremendously between countries. Parker et al. also noted that in 80% of U.S. institutions, patients or their insurance covered the cost of molecular testing, while only a small fraction (8.6%) reported that their institutions covered these expenses [5].
Another significant finding in our survey is the infrequent outsourcing of NGS (rare to never in 7/15 countries) and DNA methylation profiling (rare to never in 11/15 countries) in this region, including HICs. In these areas, the cost of advanced molecular testing is predominantly borne by the patients themselves or research grants, as it is rarely covered by insurance or government programs [7, 8]. This creates a significant financial burden, making such testing unaffordable for almost all patients, except those in the wealthy class or treated in national-center-type facilities. Further, the clinical utility of these tests for various CNS tumors remains partially understood. [9, 10] Frequently, diagnoses derived from WHO CNS5 classification using advanced molecular tests do not substantially alter treatment decisions, particularly in LMIC settings. Hence, justifying the economic investment in these tests, given their current limited clinical impact on treatment decisions, poses significant challenges, particularly in LMICs.
In addition to outsourcing, institutional twinning was another method to enhance diagnostic capabilities in LMICs and UMICs by fostering collaboration and resource exchange between institutions in these regions and HICs. For instance, collaborations involving hospitals in Pakistan (AKUH and ICCH) and Jordan (King Hussein's Cancer Center) with SickKids in Toronto, Canada, highlighted how twinning initiatives mitigate resource shortages, support capacity building, and directly benefit patients [11-13]. These programs provided critical training and mentorship for pathologists and contributed to infrastructure development by adopting and validating advanced diagnostic techniques. An additional essential advantage of institutional twinning was that associated institutions covered the costs. Thus, LMIC-HIC institutional twinning initiatives are pivotal in advancing diagnostic capabilities by leveraging expertise from HICs to enhance patient care and pathology services in resource-constrained settings sustainably.
A significant limitation of our survey is that it reflects responses only from participating centers, potentially biasing data toward institutions with more excellent resources and academic standing within each country. Non-responder data and response rates were not captured, possibly underestimating challenges, especially in LMICs. Thus, while informative, our survey findings should be interpreted considering this limitation. A second limitation of our survey is that we have not received responses from the entire group of laboratories in every country and it is possible that only larger centers/centers in bigger cities/larger hospitals where neurosurgery facilities are available could be reached through this survey. Hence it is possible that the majority of institutions that responded to our survey are probably at the higher end of the financial and academic standards of every country. This is, however, very subjective and hard to quantify, but suffice is to say that our data may “underestimate” the problems, especially in LMICs. Thirdly, there may be some unknown bias concerning the responding institutions because we do not have any information regarding the proportion of private/public/universities in these countries. Hence, we are unable to comment on whether our distribution of the types of institutions surveyed reflects the proportions expected in the respective countries. Also, we do not have exact data regarding specific neuropathology expertise, the number of pathologists in each center, the ratio of expert neuropathologists to general pathologists in these countries, or the correlation of this expertise to the level of resources, as this specific information was not collected in our survey questionnaire. Further, no statistical test of significance was applied because a random sampling approach was not utilized in the study. Also, the findings from the survey are mostly descriptive in nature and there are no hypothesis to be tested in the study.
This survey lays the foundation for the AOSNP-ADAPTR group to develop tailored guidelines aimed at enabling reasonably accurate and clinically relevant diagnoses of CNS tumors across centers and countries with varying diagnostic capabilities. Guidelines specific to each Resource Level (RL) and tumor type (such as adult-type diffuse gliomas, pediatric-type gliomas, and embryonal tumors) will be developed by individual Working Committees and rigorously reviewed by the Steering Committee. Feedback obtained during this process will be carefully considered to refine the guidelines. The revised guidelines will then undergo a 2-week open comment period to solicit input from all Advisory Board members, following which further revisions will be done. Once approved by both the Steering Committee and Advisory Board, the guidelines will be submitted to the Executive Committee of ISN. A subsequent 10 days open comment period will follow at this stage before finalizing the guidelines for publication.
5 CONCLUSIONS
In conclusion, our survey provides a comprehensive overview of current in-house/local and outsourcing resources for brain tumor diagnostics in the Asian Oceanian region, highlighting constraints and regional disparities. The limitation of in-house/local access to various diagnostic techniques was most significantly noted in LMICs, wherein the availability of surrogate IHC markers, FISH, Sanger sequencing, and NGS was a meager 57%, 29%, 10.7%, and 9.4%, respectively, in respondent centers. Another key finding of this study was the unavailability/limited availability of in-house/local access to DNA methylation profiling and the infrequent outsourcing of this technique in the region, particularly in LMICs. This critical finding is especially pertinent for the forthcoming WHO classification update as new tumor types and subtypes are being identified based on this technology.
This survey lays the foundation for the AOSNP-ADAPTR group to develop tailored and feasible guidelines to enable reasonably accurate, clinically relevant diagnoses of CNS tumors equivalent to the optimal molecular testing defined in WHO CNS5 across centers and countries with varying diagnostic capabilities. Additionally, it aims to provide tests and diagnoses that support the best current treatments for WHO tumor types within the available regional resources. Given the widespread lack of in-house/local access to advanced molecular techniques in many Asian Oceanian countries, the forthcoming recommendations from the ADAPTR group will focus on utilizing histopathology supplemented by basic and surrogate IHC markers. Simpler molecular techniques like FISH and Sanger sequencing will be recommended when necessary for diagnosis. These guidelines will be periodically updated to align with WHO revisions to promote the adoption of best practices throughout the region. The ADAPTR guidelines are expected to gain acceptance not only in the Asian Oceanian regions but also in other resource-constrained areas worldwide.
These efforts try to eventually achieve equitable access to the most promising, globally recognized treatments in the world's LMICs. Furthermore, in collaboration with the WHO Classification, ADAPTR encourages oncologists and multidisciplinary experts to use its data to advocate for improved healthcare infrastructure and workforce. The longer-term targets of ADAPTR relate to encouraging more twinning programs with countries having higher resources and ensuring the availability of RL IV and V, at least on a regional basis. This includes persuading governmental and legislative bodies to provide swift regulatory approvals and financial support for modern molecular diagnostics.
AUTHOR CONTRIBUTIONS
C. S. contributed to the study designing, analysing and interpreting the data, and writing the manuscript. S. R. equally contributed to the study design, analysis of results and drafting of the manuscript. All authors (C. S., S. R., V. S., M. A. H., S. H. P., T. T., M. E. B., H. K. N. and T. K.) contributed to conceptualising the online survey, developing the questionnaires and collecting the survey data. V. S., M. A. H., S. H. P., T. T., M. E. B., H. K. N. and T. K. provided valuable insights and edits to the manuscript. All authors approved the final version of the manuscript to be published.
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of all our colleagues from various Asian Oceanian Countries for their help in collection and editing of data for this survey (Takashi Komori [Japan], Maysa Al-Hussaini [Jordan], Tarik Tihan [Turkey], HK Ng [Hong Kong], Michael Buckland, Catriona McLean and Laveniya Satgunaseelan [Australia], Fouzia Ziad [New Zealand], Sung-Hye Park and Kim Se Hoon [Korea], Md Nowfel Islam [Bangladesh], Eka Susanto [Indonesia], Geethika Jayaweera [Sri Lanka], Amit Thapar [Nepal], Syed Ather Enam and Ahmed Gilani [Pakistan], Truong Nguyen Phan Xuan [Vietnam], Yue-Shan Piao [China], Nor Haizura Abd Rani [Malaysia], Shanop Shuangshoti [Thailand], Xuling Lin and Lai Siang Hui [Singapore], Jason Chiang [Taiwan], Vani Santosh, Geeta Chacko, Nuzhat Hussain, Rakesh Jalali and Vaishali Suri [India]—detailed information provided in Supporting Information S1). We wish to convey our special thanks to Prof. David N. Louis and Prof. Pieter Wesseling for all their advice, guidance, and support for this work. We are extremely grateful to the members of the ADAPTR Advisory Board (David N. Louis, Pieter Wesseling, Catriona A. Mclean, Rakesh Jalali, Zarnie Lwin, Girish Chinnaswamy, Yonehiro Kanemura, Sona A. Pungavkar, and Koichi Ichimura) and to all the executive members of International Society of Neuropathology (ISN) for sparing their valuable time for reviewing the manuscript and providing extremely valuable comments and suggestions.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




