The DNA Repair Component EEPD1 Regulates Actin Polymerization
Funding: This study was funded by the National Institutes of Health [R01 CA139429] and the Cancer Prevention and Research Institute of Texas [RP220269] to R.H.
ABSTRACT
Endonuclease exonuclease phosphatase domain-containing protein 1 (EEPD1) is a DNase1 superfamily member that has DNA endonuclease activity. It plays a critical role in multiple DNA repair processes such as oxidative damage repair and stressed replication fork repair. Interestingly, EEPD1 is myristoylated and palmitoylated near its amino terminus in response to high levels of cholesterol, and this localizes EEPD1 protein to the inner cell membrane. Surprisingly, we found that EEPD1 promotes cortical branching actin polymerization and proper lamellipodia formation and is necessary for subsequent cell migration. EEPD1's enhancement of actin polymerization partially required its myristoylation and palmitoylation. EEPD1 depletion also resulted in marked abnormalities in nuclear morphology. Loss of EEPD1 resulted in loss of phosphorylation of SRC, RAC1, cortactin, and profilin, which are essential steps in signaling for actin polymerization. Loss of EEPD1 lowered SRC kinase activity, which would harm actin polymerization. In summary, EEPD1 is a novel, positive regulator of the signaling pathway for actin polymerization, linking actin regulation to nuclear morphology and DNA repair.
1 Introduction
The endonuclease exonuclease phosphatase domain-containing protein 1, termed EEPD1, is a ubiquitously expressed member of the large DNase1 family of nucleases (Wu et al. 2015). We previously found that EEPD1 plays a key role in several nuclear DNA repair pathways. First, it interacts with Exo1 and recruits it to replication fork structures, where together they initiate 5′ end resection for HR-mediated repair of stressed forks and restart of replication (Kim et al. 2017). Additionally, EEPD1 has been shown to be involved in mediating synthetic lethality in RAD52-depleted BRCA1 mutant breast cancer cells by creating a cleaved replication fork intermediate that cannot progress without RAD52 (Hromas et al. 2017). We also recently discovered that EEPD1 has 5′ abasic endonuclease activity that can fully replace APE1 in the base excision repair pathway (Jaiswal et al. 2023). Depletion of EEPD1 resulted in increased cell death in the face of oxidative and alkylating DNA damage due to stressed replication fork degradation or fusion (Jaiswal et al. 2023). Consistent with these studies, miR-199a-5p represses EEPD1 levels in esophageal cancer, resulting in downregulation of the ATR/CHK1 pathway and increasing sensitivity to radiation (Phatak et al. 2023; Sun et al. 2021).
EEPD1 has also been identified as a target of liver X receptors (LXRs) and promotes cellular cholesterol efflux in macrophages by increasing ABCA1 transporter levels (Nelson et al. 2017). That report also showed that EEPD1 can localize to the plasma membrane, and this localization was dependent on its first 10 amino acids. Two EEPD1 amino acids, Gly2 and Cys7, are myristoylated and palmitoylated respectively, and these modifications were necessary for its membrane localization (Moriya et al. 2016; Nelson et al. 2017). Further, peripheral macrophages from patients with coronary arterial disease showed increased expression of miR-320b, and EEPD1 was identified as a target for this miRNA, and this resulted in attenuated cholesterol efflux from the macrophages (Lu et al. 2022).
Given that EPD1 has been found to be present at the inner cell membrane, we investigated the function of EEPD1 at the plasma membrane. EEPD1 is in the DNase1 superfamily, and DNase1 binds tightly to monomeric (G) actin, which both prevents actin polymerization and inhibits DNase1 nuclease activity (Eulitz and Mannherz 2007; Weber et al. 1994). We had previously found that depleting EEPD1 results in malformations of cell and nuclear morphology (Wu et al. 2015). For these two reasons, we tested whether one of EEPD1's roles at the inner cell membrane was to promote actin polymerization. We demonstrate here that endogenous EEPD1 is indeed expressed at the cell membrane and the loss of EEPD1 disrupts the generation of actin polymers (F-actin), which decreases cell mobility. Depletion of EEPD1 decreased SRC kinase activity, which would lower downstream signaling responsible to actin polymerization.
2 Materials and Methods
2.1 Protein Isolation and Western Blotting
For cell fractionation, proteins from cell membrane, cytoplasm, and nucleus were prepared using a subcellular fractionation kit according to the manufacturer's instructions (Fisher Scientific, Pittsburgh, PA). Protein lysates were prepared using IP lysis buffer (Fisher Scientific, Pittsburgh, PA) and western blots performed as we described (Wu et al. 2015). Protein expression changes examined using western blots were all performed in triplicate and analyzed using Image J densitometry for statistical analysis. All statistical analyses were performed using Student's t test.
2.2 Confocal Immunofluorescence
EEPD1 was visualized in A549 cells using a monoclonal anti-EEPD1 antibody (1:1000 in PBS/3% BSA) or FLAG antibody (1:1000 in PBS/3% BSA) in confocal microscopy as we described (Wu et al. 2015). To visualize F-actin, coverslips cultured with A549 cells were stained with Alexa Fluor 568 phalloidin (1 U) for 5 minat room temperature following antibody staining and washed three times with PBS before mounting on slides (Gautreau et al. 2022; Rottner et al. 2017). To visualize G-actin, coverslips were incubated with 0.3 mM DNaseI 488 conjugate for 15 min at room temperature followed by washing three times with PBS (Thermo Fisher Scientific, Waltham, MA) (Eulitz and Mannherz 2007; Weber et al. 1994). Coverslips were mounted onto slides with Fluoromount G containing DAPI. Images were analyzed by confocal microscopy. The WT and G2A/C7A EEPD1-green fluorescence protein (GFP) tagged expression vectors previously used to demonstrate that EEPD1 localized to the cell membrane (Nelson et al. 2017) did not enter the nucleus, likely due to the GFP tag, while the EEPD1-FLAG tagged expression vector localized both to the cell membrane and nucleus in the same manner as endogenous EEPD1 as assessed by our specific monoclonal antibody (Jaiswal et al. 2023; Wu et al. 2015). EEPD1 WT and G2A/C7A mutant GFP expression vectors were the generous gift of Dr. Noam Zelcer (University of Amsterdam). This species lacks the acylation sites in the amino terminal of EEPD1 and does not localize to the membrane (Moriya et al. 2016, Nelson et al. 2017). Lamin A confocal immunofluorescence microscopy as described above was used to demonstrate distortion of the nuclear envelope after EEPD1 depletion. Cortactin confocal immunofluorescent microscopy as described above was used to define lamellipodia in cells depleted of EEPD1 ∼6 h after replating. Confocal microscopy experiments were performed three distinct times. Antibodies used are detailed in Table S1. Densitometric quantification of fluorescence intensity per cell was performed using ImageJ Freehand. Statistical analyses for the fluorescence intensity comparison and the lobes/nuclei in the scrambled siRNA control and EEPD1 depleted cells were performed using unpaired, two-tailed Student's t test, and Fisher's exact test (which does not generate error deviations) was used for comparing populations of cells with lamellipodia. Otherwise, all error bars are standard error of the mean.
2.3 SRC Kinase Assay
A549 control and EEPD1-depleted protein lysates were prepared and immunoprecipitated by an antibody specific for SRC (Millipore Sigma, St. Louis, MO). Following the SRC immunoprecipitation, the samples were collected on magnetic beads and the beads washed stringently 10 times. Following this, the beads were resuspended in kinase buffer and the assay performed using the CycLex c-SRC kinase assay/inhibitor screening kit (MBL International, Schaumberg, IL). Results were visualized by measuring color formation at 450 nm. SRC kinase assays were repeated three times in at least triplicate replicates.
2.4 Cell Migration Assay
A549 cells were transfected with either scrambled siRNA (siSCR) and siEEPD1 as we described (Hromas et al. 2017; Jaiswal et al. 2023; Kim et al. 2017; Wu et al. 2015). After 24 h the medium was replaced with serum-free medium, for a premigration starvation period. Following this 24-h starvation period, the cells were harvested, resuspended in serum-free medium, counted and finally resuspended at a concentration of 2 × 105 cells per mL. 2 × 104 cells in serum-free medium was applied directly to the tissue culture treated, PET membrane insert with 8 mm pore size (Millipore Sigma, St. Louis, MO) and the insert then placed into tissue culture plates containing medium with 10% FBS (serum-rich media). The cells were then incubated for 24 h, followed by the inserts containing the migrated cells stained 0.5% crystal violet in 25% methanol. Following washing with nuclease-free deionized water the liquid is aspirated and the inserts allowed to dry for at least 16 h. Cell migration is assessed by light microscopy of the inserts to count cells attached. Migration assays were repeated at least three times in at least triplicate replicates.
3 Results
3.1 EEPD1 Localizes to Both the Inner Cell Membrane and the Nucleus
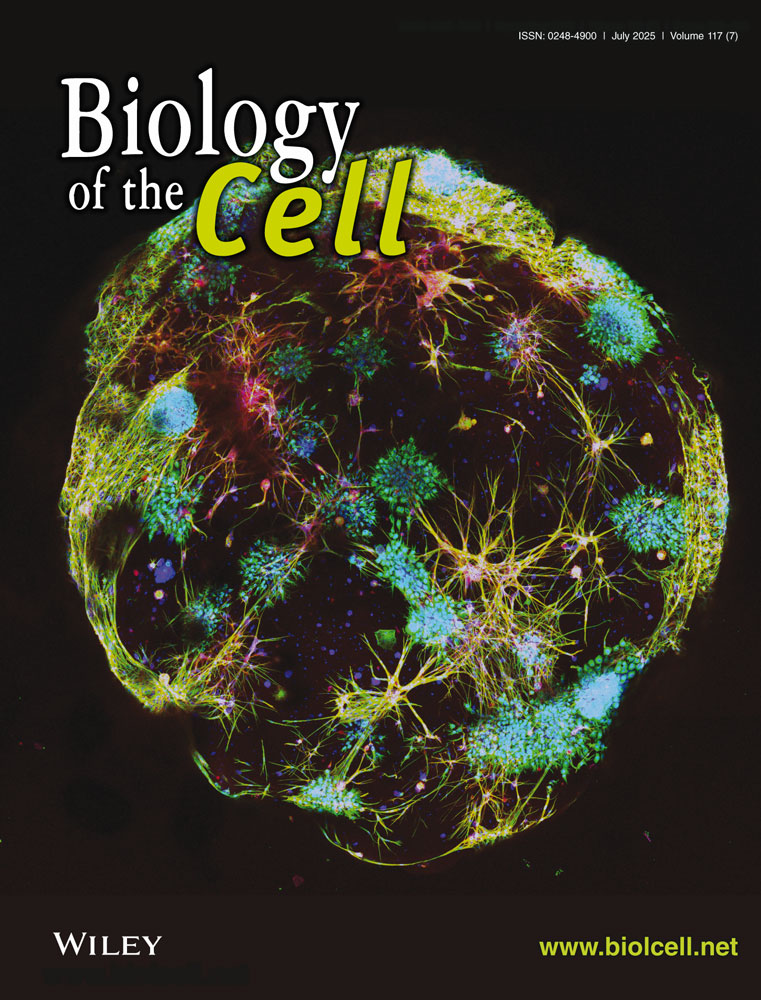
We previously demonstrated that EEPD1 is present in the nucleus using a monoclonal antibody (Jaiswal et al. 2023; Wu et al. 2015), consistent with the Protein Atlas cell localization data for EEPD1 (https://www.proteinatlas.org/ENSG00000122547-EEPD1/subcellular). Given the previous report that EEPD1 can also localize to the cell membrane via palmitoylation (at C7) and myristoylation (at G2) of its N-terminal amino acids (Nelson et al. 2017), we performed cell organelle fractionation on A549 cells. We found that EEPD1 protein has low cytoplasmic expression, but significant expression both in the membrane fraction and the nuclear fractions (Figure 1a, b). Consistent with this, the EEPD1-Flag tagged protein was also found to localize to the cell membrane and to the nucleus in confocal immunofluorescent microscopy studies (FIGURES 1, 3). Expanded photomicrographs for better representation of the EEPD1 staining are in Figure S1a.
3.2 Polymerized Actin Is Reduced in the Absence of EEPD1
Because depletion of EEPD1 results in aberrant nuclear and cell morphology (Wu et al. 2015) (Figure 1b), as well as decreasing cell plating efficiency (Hromas et al. 2017; Jaiswal et al. 2023; Kim et al. 2017; Wu et al. 2015), we examined whether EEPD1 depletion altered the actin cytoskeleton. We stained the cells with phalloidin, which binds to F-actin polymers and analyzed three cell lines using confocal microscopic immunofluorescence (Figure 1b). As shown, the loss of EEPD1 results in decreased F-actin fibers in the confocal photomicrographs in all three cell lines, indicating that this was a general phenomenon. ImageJ analysis demonstrated a statistically significant 3.4-fold decrease in phalloidin fluorescence intensity per cell in the EEPD1-depleted A549 cells compared to the scrambled control cells (Figure 1b). A higher magnification of the effect of EEPD depletion on polymerized actin is shown in Figure S1b (upper panel normal, lower panel EEPD1 depletion, red-phalloidin, green- EEPD1). Stress fibers are notably absent after EEPD1 depletion.
Actin exists in a dynamic but balanced state of polymerization and depolymerization to regulate cell shape and movement (Gautreau et al. 2022; Plessner et al. 2015; Rottner et al. 2017). These processes have several common steps; multiple membrane receptors or integrins can activate ABL1 and/or SRC, which phosphorylates RAC1/2/3 to promote activation of the WASP/WAVE complexes that initiate local actin polymerization via ARP2/3 (Arias-Salgado et al. 2003; Goly et al. 2010; Higgs and Pollard 2000; Ridley 2001; Rohatgi et al. 2000; Smith et al. 1999; Srinivasan and Plattner 2006; Tehrani et al. 2007; Zheng et al. 2023). Multiple other regulatory components interact directly with actin to regulate its polymerization or depolymerization, such as cortactin, which stabilizes Arp2/3-mediated actin branching, and cofilin which destabilizes these branches (Courtemanche and Pollard 2013; Hegelson et al. 2014; Lappalainen and Drubin 1997; Weaver et al. 2001).
We performed western blot analysis of the above signaling proteins and their posttranslational modifications (Figure 1d). When EEPD1 is depleted, SRC, RAC, cortactin, and profilin all show less phosphorylation, suggesting lower activity and thus inhibition of the actin polymerization signaling pathway, which would explain the decreased actin polymerization seen in Figure 1d, e (Arias-Salgado et al. 2003; Goly et al. 2010; Higgs and Pollard 2000; Ridley 2001; Rohatgi et al. 2000; Smith et al. 1999; Srinivasan and Plattner 2006; Tehrani et al. 2007; Zheng et al. 2023). Conversely, cofilin, a protein important for severing actin filaments into smaller fragments, shows increased phosphorylation after EEPD1 depletion (Figure 1d, e) (Arias-Salgado et al. 2003; Goly et al. 2010; Higgs and Pollard 2000; Ridley 2001; Rohatgi et al. 2000; Smith et al. 1999; Srinivasan and Plattner 2006; Tehrani et al. 2007; Zheng et al. 2023).
To confirm that the change in SRC phosphorylation was accompanied by a change in activity, we performed SRC kinase assays from protein lysates isolated from scrambled siRNA control and siEEPD1-depleted cells. As can be seen, SRC kinase activity is significantly decreased in the siEEPD1 cell lysates (Figure 1f). However, this regulation is either indirect or transient as coimmunoprecipitation assays failed to demonstrate any consistent interaction between EEPD1 and SRC (Figure 1f).
3.3 EEPD1 Is Important for New Actin Polymerization and Proper Nuclear Architecture
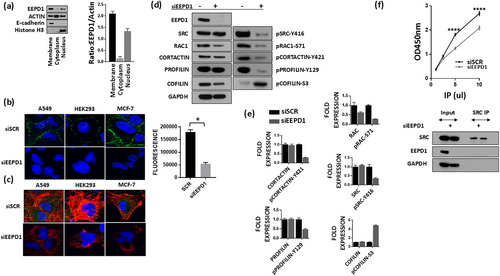
We measured new actin polymerization in EEPD1-depleted cells after trypsinization and then various timepoints after adherence to culture plates up through 48 h using confocal immunofluorescence. Cells were stained with phalloidin (F-actin) and DNase I (G-actin). As can be seen, even as early as 3 h after replating F-actin is decreased in the EEPD1-depleted cells compared to the control cells (Figure 2a). By 24 and 48 h this difference is even more pronounced (Figure 2a), with a statistically significant 3.5-fold higher mean phalloidin fluorescent intensity per cell in the scrambled siRNA control versus siEEPD1 cells after 24 h, and a statistically significant 2.9-fold higher phalloidin intensity in the scrambled control versus siEEPD1 cells after 48 h (Figure 2b). Thus, we conclude that the amount of polymerized actin in cells after removal from substrate and then readherence is inhibited when EEPD1 is absent.
Cortical actin also plays a role in maintenance of proper nuclear morphology. We previously reported that DAPI counterstaining indicated that EEPD1-depleted cells often had misshapen nuclei (Wu et al. 2015). Therefore, we stained EEPD1-depleted cells with Lamin A to assess the nuclear membrane morphology. These confocal immunofluorescent studies of the nuclear membrane found that EEPD1 loss resulted in marked alterations of nuclear shape (Figure 2c). The nuclei in the EEPD1-depleted cells were larger and developed multiple lobes. The EEPD1-depleted cells developed a statistically significant average of 2.54 lobes per nuclei while the scrambled siRNA control cells had no lobes per nuclei (Figure 2c). These data demonstrate that EEPD1 is required for maintaining proper nuclear morphology, perhaps via its promotion of actin polymerization.
Most actin regulators whose expression levels govern actin polymerization act on cortical branching actin formation. Therefore, we measured the branching actin component cortactin in lamellipodia using confocal immunofluorescence after replating scrambled siRNA control and siEEPD1-transfected cells. We found that EEPD1 depletion markedly decreased cortactin staining in the lamellipodia of these cells (Figure 2d). An average 44% of scrambled siRNA control cells had lamellipodia while 14% of cells depleted of EEPD1 had lamellipodia. In addition, the control cells had more intense staining with cortactin at the lamellipodia compared to the EEPD1-depleted cells (Figure 2d).
We next asked whether the decreased F-actin polymerization seen with EEPD1 depletion had a dysfunctional effect on cell migration to serum (Figure 2e). We found that EEPD1 depletion decreased cell migration in response to serum by three-fold, demonstrating that EEPD1 is essential for actin-mediated cell migration in response to nutrients.
3.4 Reconstitution of Full Length EEPD1 Rescues F-Actin Production
To ascertain that EEPD1 was a true regulator of actin polymerization and not an off-target effect of the siRNA, we overexpressed an FLAG-tagged full length EEPD1 without the 3′ UTR in A549 cells that had endogenous EEPD1 depleted using a siRNA against its 3′ UTR in A549 cells. We demonstrated that the transfected EEPD1 could rescue the effect that depletion of the endogenous EEPD1 had on actin polymerization (Figure 3a). We also transfected back the N-terminal RuvA domains of EEPD1 (1–267) and the C-terminal nuclease domain (200–569) into the 3′ UTR siEEPD1 cells. Restoration of either domain did not reconstitute actin polymerization when endogenous EEPD1 was depleted (Figure 3a). Scrambled control cells with the EEPD1 200–569 species transfected had a statistically significant 2.1-fold higher mean phalloidin fluorescent intensity per cell than 3′ UTR siEEPD1 cells with the EEPD1 200–569 species transfected (Figure 3a). Interestingly, the N-terminal 1–267 RuvA domain was slightly dominant negative to endogenous WT EEPD1, decreasing actin polymerization when the endogenous EEPD1 was not depleted compared to the EEPD1 200–569 species (Figure 3a). Scrambled control cells with the EEPD1 1–267 species transfected had a statistically significant 1.6-fold higher mean phalloidin fluorescent intensity per cell than 3′ UTR siEEPD1 cells with the EEPD1 1–267 species (Figure 3a), implying that the RuvA domains may partially sequester an actin polymerization component.

We then tested whether EEPD1 restoration in HeLa cells that had EEPD1 genetically deleted using CRISPR/Cas9 (Jaiswal et al. 2023) could result in reconstitution of actin polymerization. In these cells, the loss of EEPD1 again resulted in a reduction in amount of F-actin polymers and SRC Y-416 phosphorylation (Figure 3b). Confocal microscopic analysis found that the HeLa cells with EEPD1 genetically deleted had a statistically significant 3.3-fold decrease in phalloidin fluorescent intensity per cell compared to the HeLa WT EEPD1 cells (Figure 3b). Transfection of WT EEPD1 restored F-actin polymers to a pattern similar to the parental HeLa cells.
We then asked whether the localization of EEPD1 to the cell membrane was important for its actin polymerization activity. We therefore depleted endogenous EEPD1 using the 3′ UTR siRNA and transfected back EEPD1 with both the N-terminal myristoylated and palmitoylated acylation sites mutated (G2A and C7A, respectively) in A549 cells, which would abrogate membrane localization (Nelson et al. 2017). GFP confocal immunofluorescent microscopy indeed found that the EEPD1 G2A/C7A mutant species had decreased membrane localization and increased nuclear localization (Figure 3c). The mutant EEPD1 species had a statistically significant 1.7-fold decreased mean phalloidin fluorescent intensity per cell compared to scrambled siRNA control cells. However, the mutant G2A/C7A EEPD1 transfected cells after endogenous siEEPD1 depletion had a 1.5-fold higher mean phalloidin fluorescent intensity per cell than the 3′ UTR siEEPD1 depleted cells (Figure 3c). These data imply that while membrane localization is important for EEPD1's promotion of actin polymerization, it is not completely essential.
4 Discussion
EEPD1 is a DNase1 family member that has been shown to have multiple functions, including promoting HR and abasic DNA repair and subsequent restart of stressed replication forks (Hromas et al. 2017; Jaiswal et al. 2023; Kim et al. 2017; Wu et al. 2015). It also increases cholesterol efflux, and now we have demonstrated that it is a regulator of actin dynamics (Lu et al. 2022; Moriya et al. 2016; Nelson et al. 2017; Phatak et al. 2023; Sun et al. 2021). The loss of EEPD1 results in disruptions in the signaling pathway for F-actin polymerization, especially cortactin function in branching actin polymerization. Loss of EEPD1 results in decreased SRC kinase activity, which may be a possible mechanism by which EEPD1 promotes actin polymerization (Arias-Salgado et al. 2003; Smith et al. 1999; Srinivasan and Plattner 2006; Tehrani et al. 2007).
We also found that mutations in the acylation sites at G2 and C7 in EEPD1 significantly decreased actin polymerization, although not to the same extent as complete depletion of EEPD1. In addition, we found that EEPD1 is required for cell mobility in response to serum. The decrease in cortactin-stained lamellipodia after EEPD1 depletion fits with this observation (Gautreau et al. 2022; Hegelson et al. 2014; Rottner et al. 2017; Weaver et al. 2001). It may be that EEPD1 localization to the cell membrane from N-terminal acylation after exposure to sterols is required for cell mobility in response to nutrients (Nelson et al. 2017). It is possible that the localization of EEPD1 to the cell membrane exists to regulate cell movement based on the presence of environmental nutrients.
Cortical actin also plays a role in maintaining nuclear morphology (Caridi et al. 2019; Caridi et al. 2019). We found that loss of EEPD1 results in enlarged and multilobed nuclei, which may harm chromosome dynamics, such as replication, repair, and mitosis, which is consistent with another report (Caridi et al. 2019; Cheng et al. 2025). Thus, EEPD1's positive regulation of actin polymerization may contribute to cell replication in another manner besides initiation of stressed replication fork repair and restart, by maintaining nuclear structure and thus proper chromosome sequestration (Kim et al. 2017; Wu et al. 2015).
The dynamic polymerization of actin has been shown to contribute to oncogenesis (Gautreau et al. 2022; Rottner et al. 2017). Increased F-actin levels result in chemoresistance, increased cell proliferation and increased migration in neoplastic cells (Gautreau et al. 2022; Rottner et al. 2017). Thus, disruption of F-actin levels should have the opposite effect. Consistent with this, in the absence of EEPD1 we see that these cells proliferate more slowly and are more sensitive to chemotherapy (Wu et al. 2015). Therefore, EEPD1's promotion of F-actin polymerization could have clinical relevance. Targeting EEPD1 could reduce neoplastic transformation or after such transformation, EEPD1 inhibition could decrease metastasis. In that vein, we reported previously that EEPD1 depletion harms colony formation of several types of cancers (Jaiswal et al. 2023).
Although it is uncommon to observe a nuclear DNA repair protein localizing to the inner cell membrane, it has been reported for another nuclear protein; RUVBL1, a member of the AAA+ ATPases family (Taniuchi et al. 2014). RUVBL1 also binds directly to actin filaments and induces actin polymerization to produce membrane protrusions which increase the invasive properties of pancreatic cancer cells (Taniuchi et al. 2014). In addition, as mentioned above, DNase1 also binds actin, albeit with the opposite effect (Eulitz and Mannherz 2007; Weber et al. 1994). DNase1 binds to G-actin, preventing F-actin polymerization, while G-actin itself serves as an inhibitor of Dnase1 activity. It is tempting to speculate that EEPD1 and DNase1 evolved to represent a regulatory pair, one activating and the other inhibiting actin polymerization. Inhibition of actin polymerization without an alternative regulatory enhancement would be detrimental to cell survival in a rapidly changing environment (Gautreau et al. 2022; Rottner et al. 2017).
Previously we found that EEPD1 interacts with PARP1, and both proteins play a role in BER (Jaiswal et al. 2023). It is interesting to note that PARP1 is also involved in regulating cholesterol metabolism and homeostasis (Bunay et al. 2021; Shrestha et al. 2016). PARP1 has been shown to inhibit LXR-induced receptors such as LXR, which are crucial for cholesterol homeostasis (Bunay et al. 2021; Shrestha et al. 2016). Furthermore, it has also been demonstrated that PARP1 can inhibit LXR-induced ABCA1 expression and cholesterol efflux from macrophages while EEPD1 promotes both (Shrestha et al. 2016). Thus, EEPD1 appears to antagonize the effects of PARP1 on cellular cholesterol metabolism.
EEPD1's promotion of actin polymerization can explain some but not all of its genome stabilization activities. Nuclear actin is important for HR repair of DNA double strand break (DSB) damage, maintenance of nuclear morphology, generating proper cytokinesis after mitosis, and initiation of stressed replication fork repair (Caridi et al. 2019; Cheng et al. 2025; Hurst et al. 2019; Lamm et al. 2020). These are all cellular activities that EEPD1 also enhances. Indeed, nuclear actin is crucial for maintaining nuclear shape, and EEPD1 has been described as a key maintenance factor for nuclear morphology (Matsui et al. 2020; Wu et al. 2015). It is tempting to speculate that the evolutionary pressure for the differentiation of EEPD1 in the DNase1 family is this underlying enhancement of F-actin polymerization. Recent studies have found another role for nuclear actin; it can also mobilize unrepaired DNA DSBs to nuclear locations where HR is more efficient, perhaps because the entire repair complex is available (Lamm et al. 2020).
However, EEPD1's enhancement of actin polymerization cannot explain its direct action on DNA, the 5′ endonuclease function, which is documented in vitro and in vivo (Hromas et al. 2017; Jaiswal et al. 2023; Kim et al. 2017; Wu et al. 2015). Thus, EEPD1 has evolved to provide added functions that enhance genome stability beyond what would be possible with only its promotion of actin polymerization. Interestingly, EEPD1 is localized in discrete speckles in the nucleus (FIGURES 1, 3), and nuclear actin moves unrepaired DNA DSBs to discrete locations for HR repair (Matsui et al. 2020). Thus, EEPD1 could play a tripartite role in genomic stability, promoting actin polymerization at DNA damage, thereby mobilizing unrepaired DNA DSBs to nuclear locations where HR is more efficient, and cleaving damaged replication forks to initiate end resection and HR repair (Hromas et al. 2017; Jaiswal et al. 2023; Kim et al. 2017; Wu et al. 2015). The next step in these studies is to explore the connection between EEPD1's promotion of actin polymerization and its endonuclease activity in the repair of damaged DNA and stressed replication forks in vivo.
Acknowledgments
The support of the National Institutes of Health [R01 CA139429] and the Cancer Prevention and Research Institute of Texas [RP220269] to R.H. is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.