Increased relative eosinophil counts portend neck oedema after chimeric antigen receptor-T therapy
Chimeric antigen receptor (CAR)-T therapy is an effective treatment strategy for haematological malignancies. As an acute-onset adverse event, craniocervical oedema is sometimes observed right after an occurrence of cytokine release syndrome (CRS) and may cause airway obstruction.1-5 We named this phenomenon ‘neck oedema after CAR-T therapy (NEC).’ There are still very few reports, and occurrence, characteristics, mechanisms and appropriate management of NEC are still unknown. Therefore, we performed a single-centre retrospective study to describe basic NEC epidemiology and to identify a biomarker for prediction.
This study included all consecutive adult patients with relapsed or refractory B-cell malignancies received tisagenlecleucel (tisa-cel), lisocabtagene maraleucel (liso-cel), axicabtagene ciloleucel (axi-cel) or idecabtagene vicleucel (ide-cel) from 2019 to 2024 at our institute. NEC is defined as craniocervical oedema without apparent progression of a lymphoma lesion and systemic oedema observed during physical assessment by experienced physicians.1, 2, 5
We included 133 patients treated with CAR-T therapy for relapsed/refractory B-cell malignancies (Table 1). After CAR-T infusion, NEC was observed in 18 (13.5%) with a median onset timing of 3 days after CAR-T infusion (Figure S1). Of 18 with NEC, 16 (88.9%) experienced CRS 1–2 day(s) before NEC and craniocervical oedema was observed in 12 (66.7%) under administration of tocilizumab (Table S1; Figure S2), while no patient experienced craniocervical oedema under corticosteroid administration. Tocilizumab was administered for NEC in 4 (22.2%) as the treatment, and clinical symptoms did not improve in any of these cases, while symptoms improved within 2 days with corticosteroids in 16 (88.9%). Of the five cases for whom corticosteroid administration was more than 1 day after appearance of cervical oedema, NEC became more severe, and intratracheal intubation was required in two.
| Total cohort N = 133 | |
|---|---|
| Median age (range) | 63 (19–76) |
| Sex (female/male) | 70 (52.6%)/63 (47.4%) |
| Diagnosis | |
| DLBCL/FL | 107 (80.5%)/2 (1.5%) |
| B-ALL | 9 (6.8%) |
| MM | 15 (11.3%) |
| ECOG PS (0/1/2/3) | 121 (91.0%)/9 (6.8%)/2 (1.5%)/1 (0.8%) |
| CAR-T brand | |
| Tisagenlecleucel | 82 (61.7%) |
| Lisocabtagene maraleucel | 21 (15.8%) |
| Axicabtagene ciloleucel | 15 (11.3%) |
| Idecabtagene vicleucel | 15 (11.3%) |
| Treatment lines before CAR-T, median (range) | 4 (2–15) |
| Disease status at LD chemotherapy | |
| CR (or sCR)/PR (VGPR) | 27 (20.3%)/55 (41.3%) |
| SD/PD | 14 (10.5%)/28 (21.0%) |
| CRS before appearance of NEC | |
| Occurrence | 15 (83.3%) |
| Grade 1/2/3–4 | 13 (72.2%)/1 (5.6%)/1 (5.6%) |
| Treatment of CRS before appearance of NEC | |
| Tocilizumab | 12 (66.7%) |
- Abbreviations: B-ALL, acute B lymphocytic leukaemia; CAR-T, chimeric antigen receptor T cell; CR, complete remission; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; FL, follicular lymphoma; FLIPI, follicular lymphoma international prognostic index; IPI, international prognostic index; ISS, international staging system; LD chemotherapy, lympho-depletion chemotherapy; MM, multiple myeloma; NEC, neck oedema after CAR-T therapy; PD, progressive disease; PR, partial remission; sCR, stringent complete remission; SD, stable disease; VGPR, very good partial remission.
We then performed biomarker finding of NEC. Patient characteristics and blood test results were compared between patients with and without NEC. Disease status at lymphocyte depleting chemotherapy (LDC) of the NEC cohort was significantly worse than that of the non-NEC cohort (p = 0.04, Table S1). The number of cases with cervical lesions at LDC was significantly higher in the NEC cohort than the non-NEC cohort (p = 0.04, Table S1). If confined to the cohort with CRS, cervical lesions at LDC can be related to the incidence of NEC (p = 0.07, Table S2). Median lactate dehydrogenase (LDH) and soluble interleukin-2 receptor (sIL2-R) values at LDC of the NEC cohort were obviously higher than those of the non-NEC cohort (Table S3). Median values of the difference between the highest and lowest relative eosinophil counts, as well as amylase and inorganic phosphorus (iP values from Day −7 to Day 7 of CAR-T infusion of the NEC cohort were significantly larger than those of the non-NEC cohort (Table S4). Therefore, we selected disease status at LDC, high LDH and sIL-2R values at LDC, iP decrease early after CAR-T therapy, increase of eosinophil proportions and amylase through CAR-T therapy, as candidate biomarkers to predict NEC. We conducted multivariate analyses using backward stepwise selection with these six variables, along with patient age, diagnosis, CAR-T brand and cervical lesions. As a result, increased eosinophil proportion and poor disease status at LDC remained as predictors of NEC (eosinophil; hazard ratio [HR], 5.25, 95% confidential interval [CI], 2.78–10.19, p < 0.01, poor disease status; HR, 2.50, 95% CI, 1.33–4.73, p = 0.03, Table S5).
Since we found that a high ratio of eosinophils can be a predictor for NEC, we examined eosinophil proportions in patients after CAR-T therapy either with or without NEC (Figure 1A). Of 15 who experienced NEC with increased relative eosinophil counts, cervical oedema was observed 1–2 day(s) after the eosinophil increase in 14. In non-NEC patients, increased eosinophils were not observed. In non-NEC patients, the trend of eosinophil ratio was similar according to the onset of CRS (Figure 1B). The 2-year overall survival (OS) and progression-free survival (PFS) of the NEC cohort were comparable to those of the non-NEC cohort after the landmark of Day 15 (OS; HR, 1.10, p = 0.60, PFS; HR, 1.15, p = 0.29, Figure S3).
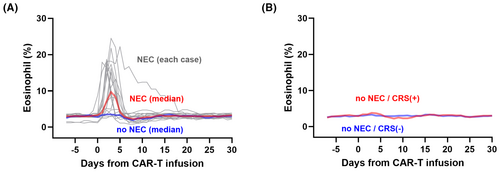
In this study, we summarized basic characteristics of NEC and identified elevation of relative eosinophil counts (>5% of total leucocytes) as a risk factor for subsequent NEC. Cervical oedema after CAR-T infusion has been reported in 2023 for the first time.6 In our cohort, intratracheal intubation was required due to severe airway obstruction in two (cases 10 and 13 in Figure S2).5 In these two cases, corticosteroid intervention was initiated more than 24 h after the appearance of craniocervical oedema. This might suggest that NEC responded rapidly to corticosteroids earlier, whereas delays in corticosteroid administration can cause patients to deteriorate, resulting in airway obstruction and requiring intubation.
Our results should be discussed from the viewpoint of cell biology. Eosinophils migrate to inflammatory lesions in the presence of interleukin-5 and bradykinin.7 Bradykinin binds to bradykinin receptors on vascular endothelial cells, which increases vascular permeability,6 resulting local or systemic oedema in hypereosinophilic diseases.8-10 In addition, increases of eosinophil proportions are associated with T-cell proliferation due to abundant production of interleukin-5 from T cells11 and are sometimes observed in patients with hypereosinophilic diseases of unknown cause.12-15 Therefore, we speculate that in cases after CAR-T infusion, expansion of CAR-T cells together with production of inflammatory cytokines, if skewed to produce more abundant interleukin-5, induces disproportionate eosinophil increases, vascular permeability and oedema. This may be why tocilizumab has little effect on NEC, whereas corticosteroids promptly affect NEC. The reason for restriction of oedema to the craniocervical region is still unknown. This study suggests that residual cervical lesions during LDC and NEC may be related, suggesting that inflammation localized at craniocervical sites may be involved in NEC pathogenesis. NEC was also observed in cases without cervical lesions during treatment courses. In some patients with multiple myeloma who did not have even extramedullary tumours, NEC was observed (case 11 in Figure S2). The mechanism of NEC is still unclear. Thus, we believe that our research can help to reveal these mysterious mechanisms.
In conclusion, NEC sometimes appears after CAR-T infusion, as a tocilizumab-refractory adverse event. Eosinophil proportion is a useful biomarker to predict NEC after CAR-T therapy, and early intervention with corticosteroids can promptly relieve symptoms.
AUTHOR CONTRIBUTIONS
NN designed the research; NN, TJ and YA organized the project and performed statistical analyses. TK, MoN, CM, JK, KY, MiN and AT-K interpreted data. All authors critically reviewed the draft and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Team CAR-T members at Kyoto University Hospital for their dedicated care of patients. This work was supported in part by research funding from Lotte Foundation, Japan awarded to YA.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
Open Research
DATA AVAILABILITY STATEMENT
Data that supports the findings of this study are available from the corresponding author upon request.




