HOPX as a tumour-suppressive protein in T-cell acute lymphoblastic leukaemia
Corresponding Author
Chien-Chin Lin
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Graduate Institute of Clinical Laboratory Sciences and Medical Biotechnology, College of Medicine, National Taiwan University, Taipei, Taiwan
Correspondence
Chien-Chin Lin and Wen-Chien Chou, Department of Laboratory Medicine, National Taiwan University Hospital, No. 7, Chung-Shan S. Rd., Taipei 10002, Taiwan.
Email: [email protected] and [email protected]
Search for more papers by this authorChia-Lang Hsu
Department of Medical Research, National Taiwan University Hospital, Taipei, Taiwan
Graduate Institute of Oncology, College of Medicine, National Taiwan University, Taipei, Taiwan
Search for more papers by this authorChi-Yuan Yao
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorYu-Hung Wang
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorChang-Tsu Yuan
Department of Pathology, National Taiwan University Hospital, Taipei, Taiwan
Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan
Department of Pathology, National Taiwan University Cancer Center, Taipei, Taiwan
Search for more papers by this authorYuan-Yeh Kuo
Tai-Cheng Stem Cell Therapy Center, National Taiwan University, Taipei, Taiwan
Search for more papers by this authorJhih-Yi Lee
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorPin-Tsen Shih
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorChein-Jun Kao
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorPo-Han Chuang
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorYueh-Chwen Hsu
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorHsin-An Hou
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorCorresponding Author
Wen-Chien Chou
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Correspondence
Chien-Chin Lin and Wen-Chien Chou, Department of Laboratory Medicine, National Taiwan University Hospital, No. 7, Chung-Shan S. Rd., Taipei 10002, Taiwan.
Email: [email protected] and [email protected]
Search for more papers by this authorHwei-Fang Tien
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology and Medical Oncology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei, Taiwan
Search for more papers by this authorCorresponding Author
Chien-Chin Lin
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Graduate Institute of Clinical Laboratory Sciences and Medical Biotechnology, College of Medicine, National Taiwan University, Taipei, Taiwan
Correspondence
Chien-Chin Lin and Wen-Chien Chou, Department of Laboratory Medicine, National Taiwan University Hospital, No. 7, Chung-Shan S. Rd., Taipei 10002, Taiwan.
Email: [email protected] and [email protected]
Search for more papers by this authorChia-Lang Hsu
Department of Medical Research, National Taiwan University Hospital, Taipei, Taiwan
Graduate Institute of Oncology, College of Medicine, National Taiwan University, Taipei, Taiwan
Search for more papers by this authorChi-Yuan Yao
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorYu-Hung Wang
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorChang-Tsu Yuan
Department of Pathology, National Taiwan University Hospital, Taipei, Taiwan
Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan
Department of Pathology, National Taiwan University Cancer Center, Taipei, Taiwan
Search for more papers by this authorYuan-Yeh Kuo
Tai-Cheng Stem Cell Therapy Center, National Taiwan University, Taipei, Taiwan
Search for more papers by this authorJhih-Yi Lee
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorPin-Tsen Shih
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorChein-Jun Kao
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorPo-Han Chuang
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorYueh-Chwen Hsu
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorHsin-An Hou
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Search for more papers by this authorCorresponding Author
Wen-Chien Chou
Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Correspondence
Chien-Chin Lin and Wen-Chien Chou, Department of Laboratory Medicine, National Taiwan University Hospital, No. 7, Chung-Shan S. Rd., Taipei 10002, Taiwan.
Email: [email protected] and [email protected]
Search for more papers by this authorHwei-Fang Tien
Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Division of Hematology and Medical Oncology, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei, Taiwan
Search for more papers by this author[Correction added on 14 February 2025, after first online publication: The subcategory has been changed.]
Summary
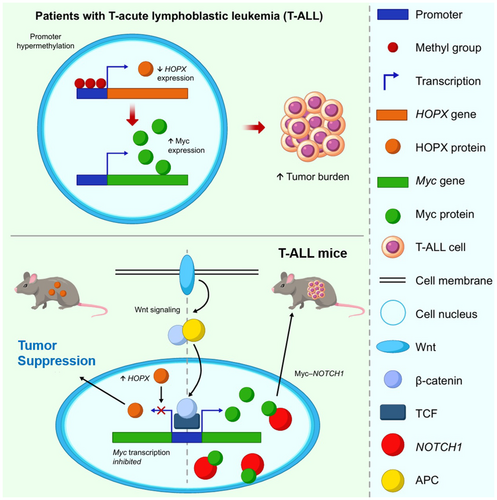
The homeodomain protein homeobox (HOPX), a multifaceted regulator of cellular functions and developmental processes, is predominantly expressed in stem cells across diverse tissues; it has also emerged as a tumour suppressor in various solid cancers. However, its role in haematological malignancies still remains undefined. This study aimed to elucidate its significance in T-cell acute lymphoblastic leukaemia (T-ALL). We firstly uncovered a novel link between reduced HOPX expression, its promoter hypermethylation and increased tumour burden in patients with T-ALL, suggesting its tumour-suppressive role. Next, we induced T-ALL by transducing intracellular NOTCH1 (ICN1) into mice with either conditional knock-in at the Rosa26 locus or knockout of Hopx. We found that T-ALL development was markedly accelerated and impeded in backgrounds with low and high Hopx expression respectively. Further analysis revealed Hopx's roles in modulating the Wnt-β-catenin pathway, a pivotal regulator of the downstream Myc signalling involved in T-ALL transformation and progression.
Graphical Abstract
We firstly uncovered a novel link between reduced HOPX expression, its promoter hypermethylation and increased tumour burden in patients with T-ALL, suggesting its tumour-suppressive role. Next, we induced T-ALL by transducing intracellular NOTCH1 (ICN1) into mice with either conditional knock-in at the Rosa26 locus or knockout of Hopx. We found that T-ALL development was markedly accelerated and impeded in backgrounds with low and high Hopx expression respectively. Further analysis revealed Hopx's roles in modulating the Wnt-β-catenin pathway, a pivotal regulator of the downstream Myc signalling involved in T-ALL transformation and progression.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
Supporting Information
| Filename | Description |
|---|---|
| bjh19965-sup-0001-DataS1.docxWord 2007 document , 21.8 MB |
Data S1. |
| bjh19965-sup-0002-TableS1-S5.xlsxExcel 2007 spreadsheet , 112.4 KB |
Tables S1–S5. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Mariotto A, Pavlova O, Park HS, Huber M, Hohl D. HOPX: the unusual homeodomain-containing protein. J Invest Dermatol. 2016; 136: 905–911.
- 2Kook H, Yung WW, Simpson RJ, Kee HJ, Shin S, Lowry JA, et al. Analysis of the structure and function of the transcriptional coregulator HOP. Biochemistry. 2006; 45: 10584–10590.
- 3Shin CH, Liu ZP, Passier R, Zhang CL, Wang DZ, Harris TM, et al. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002; 110: 725–735.
- 4Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002; 110: 713–723.
- 5Takeda N, Jain R, Leboeuf MR, Padmanabhan A, Wang Q, Li L, et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development. 2013; 140: 1655–1664.
- 6Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011; 334: 1420–1424.
- 7Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012; 31: 3079–3091.
- 8Berg DA, Su Y, Jimenez-Cyrus D, Patel A, Huang N, Morizet D, et al. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell. 2019; 177: 654–668.e615.
- 9Jain R, Li D, Gupta M, Manderfield LJ, Ifkovits JL, Wang Q, et al. HEART DEVELOPMENT. Integration of Bmp and Wnt signaling by Hopx specifies commitment of cardiomyoblasts. Science. 2015; 348:aaa6071.
- 10Chen Y, Pacyna-Gengelbach M, Deutschmann N, Niesporek S, Petersen I. Homeobox gene HOP has a potential tumor suppressive activity in human lung cancer. Int J Cancer. 2007; 121: 1021–1027.
- 11Harada Y, Kijima K, Shinmura K, Sakata M, Sakuraba K, Yokomizo K, et al. Methylation of the homeobox gene, HOPX, is frequently detected in poorly differentiated colorectal cancer. Anticancer Res. 2011; 31: 2889–2892.
- 12Yamashita K, Kim MS, Park HL, Tokumaru Y, Osada M, Inoue H, et al. HOP/OB1/NECC1 promoter DNA is frequently hypermethylated and involved in tumorigenic ability in esophageal squamous cell carcinoma. Mol Cancer Res. 2008; 6: 31–41.
- 13Katoh H, Yamashita K, Waraya M, Margalit O, Ooki A, Tamaki H, et al. Epigenetic silencing of HOPX promotes cancer progression in colorectal cancer. Neoplasia. 2012; 14: 559–571.
- 14Yamaguchi S, Asanoma K, Takao T, Kato K, Wake N. Homeobox gene HOPX is epigenetically silenced in human uterine endometrial cancer and suppresses estrogen-stimulated proliferation of cancer cells by inhibiting serum response factor. Int J Cancer. 2009; 124: 2577–2588.
- 15Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Kokubo K, et al. Potential utility of HOP homeobox gene promoter methylation as a marker of tumor aggressiveness in gastric cancer. Oncogene. 2010; 29: 3263–3275.
- 16Ren X, Yang X, Cheng B, Chen X, Zhang T, He Q, et al. HOPX hypermethylation promotes metastasis via activating SNAIL transcription in nasopharyngeal carcinoma. Nat Commun. 2017; 8:14053.
- 17Caspa Gokulan R, Yap LF, Paterson IC. HOPX: a unique homeodomain protein in development and tumor suppression. Cancers (Basel). 2022; 14:2764.
- 18Asanoma K, Matsuda T, Kondo H, Kato K, Kishino T, Niikawa N, et al. NECC1, a candidate choriocarcinoma suppressor gene that encodes a homeodomain consensus motif. Genomics. 2003; 81: 15–25.
- 19De Toni A, Zbinden M, Epstein JA, Altaba AR, Prochiantz A, Caillé I. Regulation of survival in adult hippocampal and glioblastoma stem cell lineages by the homeodomain-only protein HOP. Neural Dev. 2008; 3: 13.
- 20Chen Y, Yang L, Cui T, Pacyna-Gengelbach M, Petersen I. HOPX is methylated and exerts tumour-suppressive function through Ras-induced senescence in human lung cancer. J Pathol. 2015; 235: 397–407.
- 21Lin CC, Hsu YC, Li YH, Kuo YY, Hou HA, Lan KH, et al. Higher HOPX expression is associated with distinct clinical and biological features and predicts poor prognosis in de novo acute myeloid leukemia. Haematologica. 2017; 102: 1044–1053.
- 22Lin C-C, Yao C-Y, Hsu Y-C, Hou HA, Yuan CT, Li YH, et al. Knock-out of Hopx disrupts stemness and quiescence of hematopoietic stem cells in mice. Oncogene. 2020; 39: 5112–5123.
- 23Zhou X, Crow AL, Hartiala J, Spindler TJ, Ghazalpour A, Barsky LW, et al. The genetic landscape of hematopoietic stem cell frequency in mice. Stem Cell Rep. 2015; 5: 125–138.
- 24Bourque J, Kousnetsov R, Hawiger D. Roles of Hopx in the differentiation and functions of immune cells. Eur J Cell Biol. 2022; 101:151242.
- 25Hawiger D, Wan YY, Eynon EE, Flavell RA. The transcription cofactor Hopx is required for regulatory T cell function in dendritic cell-mediated peripheral T cell unresponsiveness. Nat Immunol. 2010; 11: 962–968.
- 26Albrecht I, Niesner U, Janke M, Menning A, Loddenkemper C, Kühl AA, et al. Persistence of effector memory Th1 cells is regulated by Hopx. Eur J Immunol. 2010; 40: 2993–3006.
- 27Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012; 122: 3398–3406.
- 28Weng AP, Ferrando AA, Lee W, Morris JP IV, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004; 306: 269–271.
- 29Tzoneva G, Ferrando AA. Recent advances on NOTCH signaling in T-ALL. Curr Top Microbiol Immunol. 2012; 360: 163–182.
- 30Li X, Gounari F, Protopopov A, Khazaie K, von Boehmer H. Oncogenesis of T-ALL and nonmalignant consequences of overexpressing intracellular NOTCH1. J Exp Med. 2008; 205: 2851–2861.
- 31Pear WS, Aster JC, Scott ML, Hasserjian RP, Soffer B, Sklar J, et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996; 183: 2283–2291.
- 32Wang YH, Lin CC, Gurashi K, Wingelhofer B, Amaral FMR, Yao CY, et al. Higher MDMX expression was associated with hypomethylating agent resistance and inferior survival in MDS patients, inferring it a potential therapeutic target. Leukemia. 2023; 37: 2507–2511.
- 33Wolock SL, Lopez R, Klein AM. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 2019; 8(281–291):e289.
- 34Germain PL, Lun A, Garcia Meixide C, Macnair W, Robinson MD. Doublet identification in single-cell sequencing data using scDblFinder. F1000Research. 2021; 10: 979.
- 35Gíslason MH, Demircan GS, Prachar M, Furtwängler B, Schwaller J, Schoof EM, et al. BloodSpot 3.0: a database of gene and protein expression data in normal and malignant haematopoiesis. Nucleic Acids Res. 2024; 52: D1138–d1142.
- 36Park JE, Botting RA, Domínguez Conde C, Popescu DM, Lavaert M, Kunz DJ, et al. A cell atlas of human thymic development defines T cell repertoire formation. Science. 2020; 367:eaay3224.
- 37Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017; 49: 1211–1218.
- 38Gekas C, D'Altri T, Aligué R, González J, Espinosa L, Bigas A. β-Catenin is required for T-cell leukemia initiation and MYC transcription downstream of Notch1. Leukemia. 2016; 30: 2002–2010.
- 39Kazanets A, Shorstova T, Hilmi K, Marques M, Witcher M. Epigenetic silencing of tumor suppressor genes: paradigms, puzzles, and potential. Biochim Biophys Acta. 2016; 1865: 275–288.
- 40Waraya M, Yamashita K, Katoh H, Ooki A, Kawamata H, Nishimiya H, et al. Cancer specific promoter CpG Islands hypermethylation of HOP homeobox (HOPX) gene and its potential tumor suppressive role in pancreatic carcinogenesis. BMC Cancer. 2012; 12: 397.
- 41Yap LF, Lai SL, Patmanathan SN, Gokulan R, Robinson CM, White JB, et al. HOPX functions as a tumour suppressor in head and neck cancer. Sci Rep. 2016; 6:38758.
- 42Ooizumi Y, Katoh H, Yokota M, Watanabe M, Yamashita K. Epigenetic silencing of HOPX is critically involved in aggressive phenotypes and patient prognosis in papillary thyroid cancer. Oncotarget. 2019; 10: 5906–5918.
- 43Sanchez-Martin M, Ferrando A. The NOTCH1-MYC highway toward T-cell acute lymphoblastic leukemia. Blood. 2017; 129: 1124–1133.
- 44Bigas A, Guiu J, Gama-Norton L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol Dis. 2013; 51: 264–270.
- 45Berquam-Vrieze KE, Swing DA, Tessarollo L, Dupuy AJ. Characterization of transgenic mice expressing cancer-associated variants of human NOTCH1. Genesis. 2012; 50: 112–118.
- 46Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012; 149: 1192–1205.
- 47Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002; 22: 1172–1183.
- 48Palpant NJ, Wang Y, Hadland B, Zaunbrecher RJ, Redd M, Jones D, et al. Chromatin and transcriptional analysis of mesoderm progenitor cells identifies HOPX as a regulator of primitive hematopoiesis. Cell Rep. 2017; 20: 1597–1608.
- 49Liang H, Wang C, Gao K, Li J, Jia R. ΜicroRNA-421 promotes the progression of non-small cell lung cancer by targeting HOPX and regulating the Wnt/β-catenin signaling pathway. Mol Med Rep. 2019; 20: 151–161.