Distinct subtypes of post-transplant lymphoproliferative disorders: CHIP-like mutations in early lesions and substantial mutational differences between EBV-positive and EBV-negative diffuse large B-cell lymphomas
[Correction added on 14 February 2025, after first online publication: The subcategory has been changed.]
Summary
Post-transplant lymphoproliferative disorders (PTLD) and lymphomas in immunocompromised individuals represent significant clinical challenges, with a limited understanding of their pathogenesis. We investigated a PTLD cohort (n = 50) consisting of ‘early lesions’ (infectious mononucleosis-like PTLD, plasmacytic and follicular hyperplasias), polymorphic PTLD and post-transplant diffuse large B-cell lymphomas (PT-DLBCL). The study also included 15 DLBCL with autoimmune/immunocompromised backgrounds (IS-DLBCL) and 14 DLBCL, not otherwise specified (DLBCL, NOS), as control. To investigate microarchitectural and genetic changes, immunohistochemistry, multiplex immunofluorescence (mIF), fluorescence in situ hybridisation and high-throughput sequencing were performed. Scarcity of viral infections other than Epstein–Barr virus (EBV) was observed. mIF revealed lower Treg infiltration in PT-DLBCL and high CD8+/PD1+ T cells in IS-DLBCL. MYC rearrangements were most common in PT-DLBCL, followed by IS-DLBCL and DLBCL, NOS, all EBV-negative. TP53 mutations were frequent in EBV-negative PT-DLBCL and DLBCL, NOS but absent in ‘early lesions’. NOTCH1 mutations were predominant in PT-DLBCL (N1 DLBCL-subgroup). Gene expression profiling showed a significant overlap between ‘early lesions’ and polymorphic PTLD. The presence of clonal haematopoiesis of indeterminate potential (CHIP)-like mutations and the absence of immune-escape gene mutations in ‘early lesions’ suggest these disorders may represent clonal expansions driven by exogenic immunosuppression and/or EBV infection ‘substituting’ for mutations of the latter group of genes.
Graphical Abstract
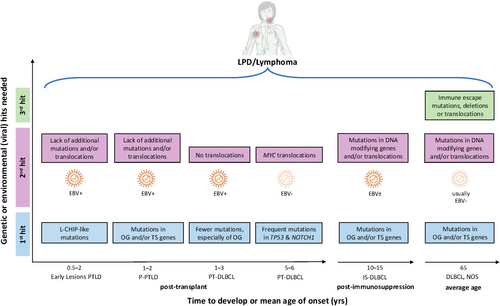
Current mechanisms of lymphomagenesis, which require at least two and/or three genetic ‘hits’ leading to the development of lymphoproliferative disease (LPD) or lymphoma. The mechanisms are shown for ‘early lesions’ post-transplant LPD (PTLD), including infectious mononucleosis (IM-), follicular hyperplasia (FH-) and plasmacytic hyperplasia (PH-) PTLDs, polymorphic (P-) PTLD, post-transplant diffuse large B-cell lymphoma (PT-DLBCL), as well as DLBCL with autoimmune/immunocompromised backgrounds (IS-DLBCL) and DLBCL, not otherwise specified (NOS). The timeline highlights the difference in time to develop PTLD, IS-DLBCL and DLBCL, NOS after transplantation, after the start of immunosuppression or the average age for the disease onset, respectively. L-CHIP, lymphoid clonal haematopoiesis of indeterminate potential; OG, oncogene; TS, tumour suppressor.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The sequencing datasets generated and analysed during this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number: PRJEB75827. Further enquiries can be directed to the corresponding author.