Acalabrutinib in combination with rituximab and lenalidomide in patients with relapsed or refractory follicular lymphoma: Results of the phase 1b open-label study (ACE-LY-003)
Summary
Patients with relapsed/refractory (R/R) follicular lymphoma (FL) have limited effective treatment options. Bruton tyrosine kinase inhibitors (BTKis) increase the anti-tumoural phenotype of tumour-associated macrophages, providing rationale to combine them with rituximab and lenalidomide (R2). Acalabrutinib, a second-generation BTKi, has potential to improve R2 efficacy without increasing T-cell–mediated toxicity due to its lack of interleukin-2–inducible T-cell kinase inhibition. Here, we report safety and efficacy from a phase 1b dose-finding study (NCT02180711) evaluating acalabrutinib plus R2 in patients with R/R FL. Overall, 29 patients received acalabrutinib plus R2 (lenalidomide 15 mg, n = 8; lenalidomide 20 mg, n = 21). At a median acalabrutinib exposure of 21 months, the most common grade ≥3 treatment-emergent adverse event (TEAE) was neutropenia (37.9%). The incidence of grade ≥3 serious TEAEs was 37.5% and 52.4% in the lenalidomide 15-mg and 20-mg cohorts, respectively; overall, the most common were COVID-19 pneumonia, COVID-19 infection and pneumonia. Earlier treatment withholdings/reductions were observed in the 20-mg cohort. With a median follow-up of 34.1 months, the overall response rate was 75.9%. The complete response rate was 25.0% and 42.9% in the lenalidomide 15- and 20-mg cohorts, respectively. Due to acceptable toxicity and preliminary efficacy, the lenalidomide 20-mg dose was selected for further investigation.
Graphical Abstract
This phase 1b open-label, dose-finding study evaluated acalabrutinib in combination with rituximab plus lenalidomide 15 or 20 mg in 29 patients with relapsed or refractory follicular lymphoma. The combination treatment had acceptable toxicity with promising clinical activity. At a median acalabrutinib exposure of 21 months, the most common grade ≥3 treatment-emergent adverse event was neutropenia, occurring in 11 (38%) patients, with no cases of febrile neutropenia. Among all treated patients, the overall response rate was 76% at a median follow-up of 34.1 months. A numerically higher complete response rate was seen in patients in the lenalidomide 20-mg group (43%) versus the 15-mg group (25%). Results from this study led to the selection of the 20-mg lenalidomide dose for further study.
INTRODUCTION
Patients with relapsed or refractory (R/R) follicular lymphoma (FL) have limited effective treatment options.1, 2 Progression of disease within 24 months (POD24) of initiating first-line chemoimmunotherapy occurs in approximately 20% of patients and is associated with relatively poor prognosis with median overall survival (OS) of approximately 5 years.3
Favourable outcomes with the anti-CD20 antibody rituximab in combination with the immunomodulatory agent lenalidomide (R2) for treatment of R/R FL have led to its widespread approval.4-6 The AUGMENT study in patients with R/R FL met its primary end-point; R2 demonstrated statistically significant and clinically relevant superiority in progression-free survival (PFS) compared with rituximab alone.6 Furthermore, the safety profile of R2 was similar to that of monotherapy with either agent and to conventional chemoimmunotherapy.7 Despite these encouraging outcomes, only 35% of patients with R/R FL achieved a complete response (CR) with R2, indicating that deeper remissions may require additional therapy.6
Studies have demonstrated that an increased number of tumour-associated macrophages (TAMs) is associated with shorter OS in patients with FL, and protumoral TAMs increase at the time of progression after front-line R2 in FL.8, 9 In vitro data show that TAMs are crucial for the survival of FL cells8 and that Bruton tyrosine kinase (BTK) inhibition increases the anti-tumoural phenotype of TAMs by affecting the crosstalk between B cells and macrophages.10, 11 These preclinical data support the combination of BTK inhibitors with rituximab or R2 for the treatment of FL. In the phase 1 Alliance A051103 study and a phase 2 study in patients with treatment-naive (TN) FL, ibrutinib plus R2 was associated with increased toxicity requiring dose modifications and discontinuations and a lack of added clinical benefit.12, 13
Acalabrutinib is a potent, selective, second-generation BTK inhibitor14 approved in the United States for the treatment of chronic lymphocytic leukaemia/small lymphocytic lymphoma and R/R mantle cell lymphoma.15 In contrast to the first-generation BTKi ibrutinib, acalabrutinib, with its specific and potent BTK inhibition and lack of interleukin-2–inducible T-cell kinase inhibition, has the potential to increase R2 efficacy without increasing T-cell–mediated toxicity.12, 16-20 This phase 1b study evaluated the safety and efficacy of acalabrutinib plus R2 in patients with R/R FL.
METHODS
Study design
Here, we report results from part 3 of a three-part, multicentre, open-label, phase 1b/2 study (NCT02180711). Results from parts 1 and 2 were previously published.21, 22 Part 3 (phase 1b) was a dose-finding study that evaluated acalabrutinib plus R2 in patients with R/R FL. Eligible patients were aged 18 years or older with documented and pathologically confirmed R/R FL (grade 1–3A) requiring treatment per National Cancer Institute or European Society for Medical Oncology guidelines. Included patients had ≥1 prior front-line standard-of-care chemoimmunotherapy or immunotherapy regimen; biopsy-confirmed residual disease was required if residual disease was suspected after prior treatment. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of two or less and radiologically measurable lymphadenopathy or extranodal lymphoid malignancy (defined as the presence of ≥1 lesions that measured ≥2.0 cm in the longest dimension and ≥1.0 cm in the longest perpendicular dimension as assessed by computed tomography [CT]). Patients with prior exposure to B-cell receptor inhibitors, lenalidomide or CAR T-cell therapy, history or presence of central nervous system lymphoma or leptomeningeal disease, clinically relevant cardiovascular disease, ongoing systemic immunosuppressive therapy, uncontrolled active systemic infections or who required treatment with proton-pump inhibitors or anticoagulants were excluded from the study.
Patients received continuous acalabrutinib 100 mg orally twice daily plus R2 in 28-day cycles until disease progression or unacceptable toxicity (Figure S1). Rituximab was administered for six cycles, followed by up to 10 additional maintenance doses for patients who had not progressed. Lenalidomide 15 or 20 mg was administered for up to 12 cycles.
A dose-limiting toxicity (DLT) review was conducted to determine the appropriate dose of lenalidomide to combine with acalabrutinib and rituximab. The DLT study design and review are described in the “Supplemental Materials” section in Supporting Information including a diagram of the enrolment and review protocol (Figure S2) and a list of DLT criteria (Table S1). At the end of cycle 1, patients were evaluated for DLTs on a rolling basis. A total enrolment of approximately 26–32 patients was planned for the study.
The study was performed in accordance with the protocol, the International Conference on Harmonisation/Guidelines for Good Clinical Practice, applicable local regulations and the ethical principles established in the Declaration of Helsinki. The protocol was approved by each site's investigational review board/independent ethics committee. All patients provided written informed consent.
Outcomes and assessments
The primary end-point was safety. Secondary end-points included investigator-assessed overall response rate (ORR) per Lugano criteria,23 duration of response (DOR), PFS and OS. Baseline disease was assessed by bone marrow aspirate and biopsy, CT with contrast and positron emission tomography (PET)/CT within 90, 30 and 60 days, respectively, before the first dose of study drug. During treatment, CT was performed for tumour assessments every three cycles starting at day 1 of cycle 4, 7, 10 and 13, and then every 24 weeks thereafter or more frequently at the investigator's discretion. PET/CT was performed on day 1 of cycles 4 and 7, and to confirm CR. Bone marrow aspirate/biopsy (if involved by lymphoma at baseline) and PET/CT were required to confirm CR. Molecular profiling of circulating tumour DNA (ctDNA) was performed as an exploratory analysis (see Table S2 for methodological details).
Statistical analysis
Descriptive statistics were used to summarize data, including mean, standard deviation and median for continuous variables and proportion for discrete variables. Safety and efficacy analyses were performed in the all-treated population, defined as all enrolled patients who received at least one dose of study drug. The efficacy-evaluable population includes all patients in the all-treated population who had at least one response assessment after the first dose of any study drug.
ORR was defined as the proportion of patients who achieved a CR or partial response (PR) per Lugano criteria23 and was calculated with its corresponding two-sided 95% confidence interval. DOR was defined as the time from the first documentation of CR or PR to the earlier of the first documentation of definitive disease progression or death from any cause. PFS was defined as the time from the start of any study drug to the earlier of the first documentation of objective disease progression or death from any cause. Patients who did not have disease progression or death were censored at the date of last tumour assessment and before initiation of subsequent anticancer therapy. Duration of OS was measured from the start of any study drug until the date of death from any cause. Surviving patients were censored at their last contact date. Kaplan–Meier methods were used to estimate DOR, PFS and OS curves and corresponding medians.
RESULTS
Patients
As of the data cut-off (25 August 2023), 29 patients with R/R FL were enrolled and received acalabrutinib plus R2; eight patients were treated with lenalidomide 15 mg (lenalidomide 15-mg cohort) and 21 patients were treated with lenalidomide 20 mg (lenalidomide 20-mg cohort). Baseline demographics and characteristics are shown in Table 1. Patients in the lenalidomide 15- and 20-mg cohorts had a median number of 2 and 1 prior therapies, respectively; 10 (34.5%) patients were refractory to last treatment and 14 (48.3%) patients were refractory to a prior anti-CD20 antibody regimen. The majority of patients (72.4%, n = 21) had intermediate or high Follicular Lymphoma International Prognostic Index (FLIPI) score. Overall, 69% of patients (n = 20) had high tumour burden per Groupe d'Etude des Lymphomas Folliculaires (GELF) criteria, 69% of patients (n = 20) had extranodal involvement and 34% of patients (n = 10) had bone marrow involvement. POD24 data were not available.
| Acalabrutinib + rituximab + lenalidomide 15 mg | Acalabrutinib + rituximab + lenalidomide 20 mg | Total | |
|---|---|---|---|
| (n = 8) | (n = 21) | (n = 29) | |
| Age, median (range), years | 65 (51–76) | 64 (44–75) | 64 (44–76) |
| Age ≥65 years, n (%) | 4 (50.0) | 10 (47.6) | 14 (48.3) |
| Sex, n (%) | |||
| Male | 7 (87.5) | 15 (71.4) | 22 (75.9) |
| Female | 1 (12.5) | 6 (28.6) | 7 (24.1) |
| Race, n (%) | |||
| Asian | 0 | 1 (4.8) | 1 (3.4) |
| Black or African American | 0 | 2 (9.5) | 2 (6.9) |
| White | 7 (87.5) | 17 (81.0) | 24 (82.8) |
| Not reported | 1 (12.5) | 1 (4.8) | 2 (6.9) |
| ECOG performance status, n (%) | |||
| 0 | 6 (75.0) | 12 (57.1) | 18 (62.1) |
| 1 | 2 (25.0) | 9 (42.9) | 11 (37.9) |
| Ann Arbor stage at baseline, n (%) | |||
| I–II | 1 (12.5) | 1 (4.8) | 2 (6.9) |
| III–IV | 7 (87.5) | 20 (95.2) | 27 (93.1) |
| Follicular lymphoma grade, n (%) | |||
| 1 | 3 (37.5) | 5 (23.8) | 8 (27.6) |
| 2 | 3 (37.5) | 10 (47.6) | 13 (44.8) |
| 3A | 2 (25.0) | 4 (19.0) | 6 (20.7) |
| Unknown | 0 | 2 (9.5) | 2 (6.9) |
| Tumour bulk, n (%) | |||
| ≥5 cm | 2 (25.0) | 11 (52.4) | 13 (44.8) |
| ≥10 cm | 1 (12.5) | 1 (4.8) | 2 (6.9) |
| FLIPI score,a n (%) | |||
| Low (0–1) | 2 (25.0) | 6 (28.6) | 8 (27.6) |
| Intermediate (2) | 4 (50.0) | 8 (38.1) | 12 (41.4) |
| High (3–5) | 2 (25.0) | 7 (33.3) | 9 (31.0) |
| Bone marrow involvement, n (%) | 3 (37.5) | 7 (33.3) | 10 (34.5) |
| Extranodal disease, n (%) | 6 (75.0) | 14 (66.7) | 20 (69.0) |
| High tumour burden per GELF criteria,b n (%) | 4 (50.0) | 16 (76.2) | 20 (69.0) |
| Elevated LDH, n (%) | |||
| LDH > ULN | 1 (12.5) | 6 (28.6) | 7 (24.1) |
| Prior systemic regimens,c n (%) | |||
| 1 | 3 (37.5) | 12 (57.1) | 15 (51.7) |
| 2 | 5 (62.5) | 4 (19) | 9 (31) |
| ≥3 | 0 | 5 (23.8) | 5 (17.2) |
| Prior systemic regimens,c median (range) | 2.0 (1–2) | 1.0 (1–5) | 1.0 (1–5) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; FLIPI, Follicular Lymphoma International Prognostic Index; GELF, Groupe d'Etude des Lymphomes Folliculaires; LDH, lactate dehydrogenase; ULN, upper limit of normal.
- a FLIPI score is defined as the number of following factors that are true: age >60, Ann Arbor stage >3, haemoglobin <12 g/dL, concentration of serum LDH > ULN and number of nodal areas >4.
- b High tumour burden is defined as having one or more of the following GELF criteria: target lesion >7 cm in diameter, three nodal target lesions >3 cm in diameter each, any B symptom, serum LDH > ULN, serum haemoglobin ≤100 g/L, neutrophil count ≤1500 cells/μL or platelet count ≤100 000 platelets/mL.
- c Excludes maintenance, consolidation, adjuvant and neoadjuvant therapies when reported on the case report form as a separate record.
Safety
At the data cut-off, the overall median (range) duration of exposure to acalabrutinib was 21 (0.5–48.2) months. All patients experienced at least one treatment-emergent adverse event (TEAE) of any grade. Grade ≥3 TEAEs occurring in at least two patients and events of clinical interest are presented in Table 2. Overall, most patients (79.3%, n = 23) experienced at least 1 grade ≥3 TEAE; the incidence of these TEAEs was 50% (n = 4) in the lenalidomide 15-mg cohort and 90.5% (n = 19) in the lenalidomide 20-mg cohort. The most common grade ≥3 TEAEs were neutropenia (37.9%, n = 11), COVID-19 pneumonia (13.8%, n = 4), COVID-19 infection (10.3%, n = 3) and other pneumonias (10.3%, n = 3). No instances of febrile neutropenia were reported. The incidence of grade ≥3 serious AEs was 37.5% and 52.4% in the lenalidomide 15- and 20-mg cohorts, respectively. Overall, the most common grade ≥3 serious AEs were COVID-19 pneumonia (13.8%, n = 4), COVID-19 infection (10.3%, n = 3) and other pneumonias (10.3%, n = 3).
| N (%) | Acalabrutinib + rituximab + lenalidomide 15 mg | Acalabrutinib + rituximab + lenalidomide 20 mg | Total | |||
|---|---|---|---|---|---|---|
| (n = 8) | (n = 21) | (n = 29) | ||||
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any TEAE | 8 (100) | 4 (50.0) | 21 (100) | 19 (90.5) | 29 (100) | 23 (79.3) |
| Grade ≥3 TEAEsa occurring in ≥2 patients in any cohort | ||||||
| Neutropenia/neutrophil count decreased | 1 (12.5) | 1 (12.5) | 14 (66.7) | 10 (47.6) | 15 (51.7) | 11 (37.9) |
| COVID-19 pneumonia | 0 | 0 | 4 (19.0) | 4 (19.0) | 4 (13.8) | 4 (13.8) |
| COVID-19 | 1 (12.5) | 1 (12.5) | 7 (33.3) | 2 (9.5) | 8 (27.6) | 3 (10.3) |
| Pneumonia | 0 | 0 | 4 (19.0) | 3 (14.3) | 4 (13.8) | 3 (10.3) |
| Lymphocyte count decreased | 0 | 0 | 2 (9.5) | 2 (9.5) | 2 (6.9) | 2 (6.9) |
| Rash | 2 (25.0) | 0 | 5 (23.8) | 2 (9.5) | 7 (24.1) | 2 (6.9) |
| Sepsis | 0 | 0 | 2 (9.5) | 2 (9.5) | 2 (6.9) | 2 (6.9) |
| Thrombocytopenia | 1 (12.5) | 0 | 2 (9.5) | 2 (9.5) | 3 (10.3) | 2 (6.9) |
| White blood cell count decreased | 0 | 0 | 3 (14.3) | 2 (9.5) | 3 (10.3) | 2 (6.9) |
| Grade 5 TEAE | 0 | 0 | 1 (4.8)b | 1 (4.8)b | 1 (3.4)b | 1 (3.4)b |
| Serious TEAE | 4 (50.0) | 3 (37.5) | 11 (52.4) | 11 (52.4) | 15 (51.7) | 14 (48.3) |
| Events of clinical interesta | ||||||
| Cardiac events | 3 (37.5) | 1 (12.5) | 6 (28.6) | 2 (9.5) | 9 (31) | 3 (10.3) |
| Atrial fibrillation | 1 (12.5) | 0 | 1 (4.8) | 1 (4.8) | 2 (6.9) | 1 (3.4)c |
| Ventricular tachyarrhythmias | 0 | 0 | 0 | 0 | 0 | 0 |
| Anaemia | 2 (2.5) | 1 (12.5) | 2 (9.5) | 0 | 4 (13.8) | 1 (3.4) |
| Leucopenia | 1 (12.5) | 1 (12.5) | 17 (81) | 13 (61.9) | 18 (62.1) | 14 (48.3) |
| Neutropenia | 1 (12.5) | 1 (12.5) | 14 (66.7) | 10 (47.6) | 15 (51.7) | 11 (37.9) |
| Other leucopenia | 0 | 0 | 6 (28.6) | 5 (23.8) | 6 (20.7) | 5 (17.2) |
| Thrombocytopenia | 1 (12.5) | 0 | 4 (19.0) | 2 (9.5) | 5 (17.2) | 2 (6.9) |
| Haemorrhage | 1 (12.5) | 0 | 11 (52.4) | 0 | 12 (41.4) | 0 |
| Major haemorrhage | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatotoxicity | 0 | 0 | 2 (9.5) | 0 | 2 (6.9) | 0 |
| Hypertension | 0 | 0 | 2 (9.5) | 0 | 2 (6.9) | 0 |
| Infections | 5 (62.5) | 2 (25) | 15 (71.4) | 10 (47.6) | 20 (69) | 12 (41.4) |
| Interstitial lung disease/pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 |
| Second primary malignancies | 0 | 0 | 1 (4.8) | 1 (4.8) | 1 (3.4) | 1 (3.4) |
| Secondary primary malignancies, excluding non-melanoma skin cancer | 0 | 0 | 1 (4.8) | 1 (4.8) | 1 (3.4) | 1 (3.4) |
| Tumour lysis syndrome | 0 | 0 | 1 (4.8) | 1 (4.8) | 1 (3.4) | 1 (3.4) |
- Abbreviations: AE, adverse event; FL, follicular lymphoma; TEAE, treatment-emergent adverse event.
- a A patient with multiple grades for a given AE was counted only once under the highest grade.
- b Death due to sepsis occurred 6 days after treatment was interrupted for grade 4 neutropenia.
- c Patient experienced grade 3 atrial flutter.
Among AEs of clinical interest, atrial fibrillation was reported in one patient each (both grade 2) in the lenalidomide 15- and 20-mg cohorts; neither patient had a medical history of atrial fibrillation. Hypertension as an AE of clinical interest was reported in two patients (grade 1 and grade 2) in the lenalidomide 20-mg cohort; the patient with the grade 1 event had new-onset hypertension and the patient with the grade 2 event had a prior history of hypertension. No ventricular arrythmias or sudden cardiac deaths were reported.
One patient in the lenalidomide 20-mg cohort experienced a grade 5 TEAE of sepsis and died 7 days after the last dose of acalabrutinib. Three other deaths occurred more than 30 days after the last dose of acalabrutinib; in the 15-mg cohort, one patient died due to septicaemia, and in the 20-mg cohort, one patient died due to aortic aneurysm and another was lost to follow-up resulting in unknown cause of death.
A summary of acalabrutinib, rituximab and lenalidomide discontinuations and dose modifications is presented in Table 3. In the lenalidomide 15-mg cohort, acalabrutinib dose withholdings due to AEs occurred in 2 (25.0%) patients; no patient had acalabrutinib dose reductions or lenalidomide withholdings or reductions due to AEs. In the lenalidomide 20-mg cohort, acalabrutinib dose withholdings and reductions due to AEs occurred in 8 (38.1%) patients and 1 (4.8%) patient, respectively; lenalidomide dose withholdings and reductions due to AEs occurred in 7 (33.3%) patients each. The median (range) time to first dose withholding was 401 (20–862) days for acalabrutinib and 94 (14–174) days for lenalidomide in the lenalidomide 15-mg cohort. The median (range) time to first dose withholding was 71.5 (2–408) days for acalabrutinib and 33.5 (1–282) days for lenalidomide in the lenalidomide 20-mg cohort. In the lenalidomide 15- and 20-mg cohorts, dose discontinuations due to AEs were 25.0% and 23.8% for acalabrutinib, 25.0% and 19.0% for rituximab and 12.5% and 19.0% for lenalidomide, respectively.
| Acalabrutinib + rituximab + lenalidomide 15 mg | Acalabrutinib + rituximab + lenalidomide 20 mg | Total | |
|---|---|---|---|
| (n = 8) | (n = 21) | (n = 29) | |
| Time on study, median (range), months | 29.3 (1.9–54.3) | 34.1 (0.7–46.1) | 34.1 (0.7–54.3) |
| Duration of exposure to acalabrutinib, median (range), months | 18.1 (0.6–48.2) | 23 (0.5–44.3) | 21 (0.5–48.2) |
| Discontinued study, n (%) | 8 (100) | 21 (100) | 29 (100) |
| Withdrawal of consent | 3 (37.5) | 2 (9.5) | 5 (17.2) |
| Death | 1 (12.5) | 3 (14.3) | 4 (13.8) |
| Patient lost to follow-up | 0 | 3 (14.3) | 3 (10.3) |
| Enrolled into follow-up studya | 4 (50.0) | 13 (61.9) | 17 (58.6) |
| Acalabrutinib dose modificationsb | |||
| Dose withholding,c n (%) | 3 (37.5) | 12 (57.1) | 15 (51.7) |
| Due to AE, n (%) | 2 (25.0) | 8 (38.1) | 10 (34.5) |
| Time to first dose withholding,c median (range), days | 401 (20–862) | 71.5 (2–408) | 72.0 (2–862) |
| Dose reduction,d n (%) | 0 | 3 (14.3) | 3 (10.3) |
| Due to AE, n (%) | 0 | 1 (4.8) | 1 (3.4) |
| Time to first dose reduction,d median (range), days | – | 225 (129–390) | 225 (129–390) |
| Dose discontinuation due to AE, n (%) | 2 (25.0) | 5 (23.8) | 7 (24.1) |
| Lenalidomide dose modificationsb | |||
| Dose withholding,c n (%) | 2 (25.0) | 10 (47.6) | 12 (41.4) |
| Due to AE, n (%) | 0 | 7 (33.3) | 7 (24.1) |
| Time to first dose withholding,c median (range), days | 94 (14–174) | 33.5 (1–282) | 33.5 (1–282) |
| Dose reduction,d n (%) | 0 | 8 (38.1) | 8 (27.6) |
| Due to AE, n (%) | 0 | 7 (33.3) | 7 (24.1) |
| Time to first dose reduction,d median (range) days | – | 42 (1–294) | 42 (1–294) |
| Dose discontinuation due to AE, n (%) | 1 (12.5) | 4 (19.0) | 5 (17.2) |
| Rituximab dose modificationsb | |||
| Dose interruption,e n (%) | 3 (37.5) | 9 (42.9) | 12 (41.4) |
| Time to first dose interruption,e median (range), days | 1 (1–1) | 1 (1–88) | 1 (1–88) |
| Dose reduction,f n (%) | 1 (12.5) | 1 (4.8) | 2 (6.9) |
| Time to first dose reduction,f median (range) days | 59 (59–59) | 15 (15–15) | 37 (15–59) |
| Dose discontinuation due to AE, n (%) | 2 (25.0) | 4 (19.0) | 6 (20.7) |
- Abbreviation: AE, adverse event.
- a Patients still on treatment at the date of final data cut-off, and continuing to derive clinical benefit in the investigator's opinion were transitioned to AstraZeneca's Post Trial Access Program phase of the study.
- b A patient was counted once for each reason for dose withholding, interruption or reduction even though a patient may have experienced the reason multiple times throughout the dosing period.
- c Dose withholding was defined as missing dose for ≥7 consecutive dosing days (for lenalidomide: days 22–28 of each cycle are not considered as they are not dosing days).
- d Dose reduction was defined as taking lower dose level of acalabrutinib (100 mg daily) or lower than assigned dose level of lenalidomide for ≥3 consecutive dosing days (for lenalidomide: days 22–28 of each cycle are not considered as they are not dosing days).
- e Dose interruption was based on the data collected on the case report form: ‘Was the infusion interrupted?’ = Yes.
- f Dose reduction was based on the data from the dose administration case report form: ‘If dose administered is less than calculated dose, specify reason’ = non-missing.
Efficacy
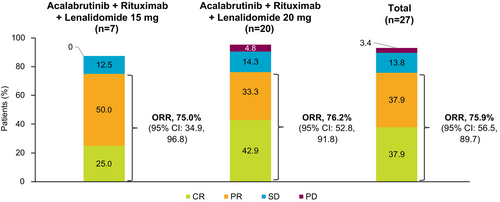
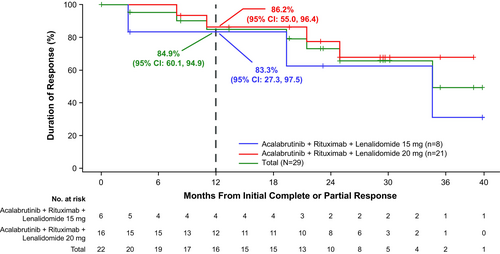
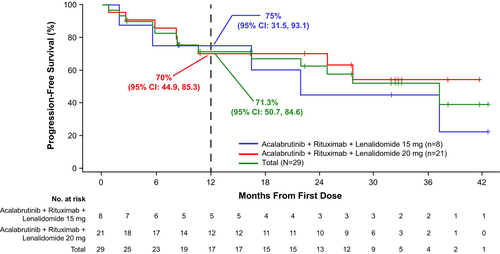
Nearly all patients in the lenalidomide 15-mg cohort (7 of 8) and 20-mg cohort (20 of 21) were evaluable for response; one patient in each cohort had missing response assessments. In the lenalidomide 15-mg cohort, ORR was 75.0% (CR, 25.0%; PR, 50.0%); one patient (12.5%) had stable disease (SD) (Figure 1). ORR in the lenalidomide 20-mg cohort was 76.2% (CR, 42.9%; PR, 33.3%); three patients (14.3%) had SD and one patient (4.8%) had progressive disease. With a median follow-up of 29.3 months in the lenalidomide 15-mg cohort, the median DOR was 34.5 months, median PFS was 21.9 months and median OS was not reached (NR); 12-month DOR, PFS and OS estimates were 83.3%, 75.0% and 87.5%, respectively (Figures 2-4). With a median follow-up of 34.1 months in the lenalidomide 20-mg cohort, the median DOR, median PFS and median OS were NR; 12-month DOR, PFS and OS estimates were 86.2%, 70.0% and 90.2%, respectively.

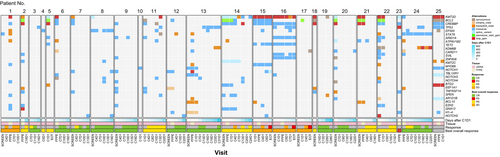
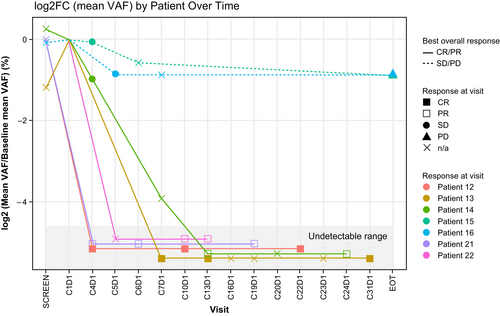
Genomic analysis of formalin-fixed paraffin-embedded (FFPE) biopsy samples and/or cell-free DNA from peripheral blood was performed for 25 patients. The most frequently altered genes included KMT2D, CREBBP and BCL2 (Figure 5). Among FFPE samples, no genes were identified that were mutated significantly more frequently among responders (those who achieved CR/PR) versus non-responders. Seven patients had trackable variants meeting the cut-off criteria. Of these, two patients with SD maintained a consistent mean variant allele frequency (VAF) in ctDNA during treatment (Figure 6; Figure S3) while five patients with CR or PR demonstrated decreased mean VAF over time; many gene variants detected at baseline fell to undetectable levels following treatment (Figure S4).
DISCUSSION
In this phase 1b, open-label study of patients with R/R FL, acalabrutinib plus R2 showed clinical activity with an ORR of 75.9% and CR rate of 37.9% in the all-treated population at a median follow-up of 34.1 months. Although patient numbers were small, the CR rate in the lenalidomide 20-mg cohort (42.9%) was higher than in the 15-mg cohort (25.0%), suggesting a better quality of response. Baseline characteristics were reasonably matched between the two cohorts, although the proportion of patients with only one prior line of therapy trended higher in the 20-mg cohort (57% vs. 38%), potentially favouring outcomes in this group. Conversely, a trend towards higher tumour burden per GELF criteria in the 20-mg cohort (76% vs. 50%) may have favoured outcomes in the 15-mg cohort. We therefore cannot exclude the possibility that differences in the composition of each cohort contributed to the observed outcome differences. The regimen had acceptable toxicity, consistent with the known safety profiles of the individual drugs.
The phase 3 AUGMENT study demonstrated a significant improvement in PFS associated with the combination of lenalidomide 20 mg plus rituximab compared with rituximab monotherapy in patients with R/R FL.6 Although the number of patients in our study was small and the study populations may differ (i.e. prior treatments), in both studies, approximately 50% of patients had only one prior systemic anticancer therapy and most patients had a FLIPI score of 0–2. In this context, the efficacy of the combination of acalabrutinib plus R2 was encouraging. In the lenalidomide 20-mg cohort, at a follow-up of 34.1 months, median PFS (investigator-assessed) was NR (39-month PFS estimate was 54.0%) compared with a median investigator-assessed PFS of 25.3 months (39.4 months by independent review committee) for R2 in the AUGMENT study. Grade ≥3 neutropenia occurred in 37.9% of patients in our study. By comparison, the incidence of grade ≥3 neutropenia with R2 therapy was 50% in the AUGMENT study (median follow-up, 28.3 months) and 20% in patients with R/R FL in the CALGB 50401 study (median follow-up, 2.5 years).6, 24 Taken together, and with the caveat that our study was small and that cross-trial comparisons can be misleading, these results suggest that addition of acalabrutinib to R2 may result in durable remissions in patients with R/R FL without significantly increasing toxicity.
We have previously reported that acalabrutinib monotherapy and acalabrutinib plus rituximab in R/R FL both demonstrated relatively limited efficacy, with ORRs of 33.3% and 30.8%, respectively, and median PFS of 12.0 and 8.3 months.22 By comparison, our results suggest that addition of lenalidomide to acalabrutinib and rituximab increases both ORR and PFS in this patient population. Although haematological toxicity, in particular neutropenia, was relatively common with the acalabrutinib-R2 combination, no instances of febrile neutropenia were reported, suggesting that the recommended management guidelines (including dose modifications and use of haematopoietic growth factors) were effective in mitigating the clinical impact of this toxicity. Notably, the safety profile of the acalabrutinib-R2 regimen may have been influenced by the timing of the study during the COVID-19 pandemic as COVID-19 pneumonia (13.8%) and COVID-19 infection (10.3%) were the most common grade ≥3 TEAEs after neutropenia.
The combination of ibrutinib plus R2 in patients with TN FL was examined in the phase 1 Alliance A051103 study.12 Favourable clinical efficacy was observed with an ORR of 95% and estimated 12-month PFS of 80%. Similar efficacy outcomes were seen in a phase 2 study of ibrutinib-R2 in patients with TN FL and MZL (ORR of 95.8% and estimated 24-month PFS of 78.8%).13 The higher activity observed in these ibrutinib studies compared to our study (ORR of 75.9% and 12-month PFS of 71.3%) is likely attributed to differences in patient populations (TN vs. R/R FL). Despite the high response rates with ibrutinib-R2, 82% of patients in the Alliance A051103 study developed a rash (grade ≥3, 36%) and 83.3% of patients in the phase 2 study experienced a rash (grade ≥3, 50%). By comparison, the incidence of rash in our study was much lower (24% and 7% for any grade and grade ≥3, respectively), possibly reflecting the greater specificity of acalabrutinib for BTK.
Genomic analysis in this study demonstrated decreased mean VAF in ctDNA following treatment among patients who achieved CR or PR. Molecular assays currently available for the detection of minimal residual disease, including digital polymerase chain reaction, Clono-SEQ® and cancer personalized profiling by deep sequencing (CAPP-Seq), have multiple limitations in FL.25, 26 Data supporting the use of phased variant enrichment and detection by sequencing (PhasED-Seq) in FL are eagerly awaited and may help identify patient subsets for whom immunotherapy could be curative.
Study limitations include small sample size, including limited number of tumour samples available for genomic analysis; lack of a control group; and no available data on POD24 status. Additionally, correlative analyses to identify potential mechanisms of resistance and response to the acalabrutinib–R2 combination were not available.
The emergence of novel-targeted agents has evolved the treatment landscape for FL. In the ROSEWOOD study, patients with R/R FL who received zanubrutinib plus obinutuzumab had an ORR of 69% (CR rate, 39%) and median PFS of 28.0 months at a median follow-up of 20.2 months.27 These results led to the approval of this combination and were the basis for the ongoing phase 3 MAHOGANY study evaluating zanubrutinib and obinutuzumab versus R2 in patients with R/R FL.28, 29 Other novel agents including two CD19 chimeric antigen receptor T-cell products, axicabtagene ciloleucel30 and tisagenlecleucel31; two CD3/CD20 bispecific antibodies, mosunetuzumab32 and epcoritamab33; and the EZH2 inhibitor, tazemetostat,34 have recently been approved for the treatment of FL in the R/R setting, further emphasizing the need to define optimal treatment selection.
In conclusion, acalabrutinib, a second-generation BTK inhibitor, in combination with R2 had acceptable toxicity with promising clinical activity. Based on the results of this study, the lenalidomide 20-mg dose was selected for further exploration. A phase 2 investigator-initiated study evaluating acalabrutinib plus R2 in patients with TN FL is ongoing (NCT04404088).
AUTHOR CONTRIBUTIONS
RA, ISL, MC, RK, DMS and PS served as study investigators. RA, ISL, MC, RK, DMS and PS enrolled patients. RL and C-CW conducted data analysis. All authors interpreted data, reviewed and revised the manuscript and provided final approval of the manuscript.
ACKNOWLEDGEMENTS
The study was sponsored by AstraZeneca. Medical writing assistance, funded by AstraZeneca, was provided by Amy Knapp, PhD, and Sarah Huh, PharmD, of Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors. The authors thank Rachel Kositsky, PhD, for assistance in data analysis and Yi Li, PhD, for assistance in statistical analysis.
CONFLICT OF INTEREST STATEMENT
Paolo Strati: Consultancy: Kite-Gilead, Roche-Genentech, Hutchinson MediPharma, ADC Therapeutics, Incyte–Morphosis, Ipsen, AbbVie and Genmab; Research Funding: Sobi Pharmaceuticals, AstraZeneca/Acerta, ALX Oncology and ADC Therapeutics; Salary Support: Leukemia Lymphoma Society Scholar in Clinical Research Career Development Program, Sabin Fellowship Award and Gilead Scholar in Clinical Research Award. Richy Agajanian: None to disclose. Izidore S. Lossos: Honoraria: Adaptive; Research Funding: NCI; Membership on an Entity's Board of Directors or Advisory Committees: LRF. Morton Coleman: Research Funding: Arcus Biosciences, AstraZeneca, Acerta Pharma, BeiGene, BMS, Lilly, EMD Serono, Genentech/Roche, GenFleet, GSK, Hutchison MediPharma, Incyte, Ipsen, MEI Pharma, Merck, Napo, Novartis, Pfizer and Seattle Genetics; Consultancy: BMS. Robert Kridel: Research Funding: AbbVie, Roche. Andrew Wood: Employment: AstraZeneca. Robin Lesley: Employment: AstraZeneca. Chuan-Chuan Wun: Employment: AstraZeneca. Deborah M. Stephens: Consultancy: AbbVie, AstraZeneca, Celgene, BeiGene, Lilly, Genentech, Janssen, Pharmacyclics; Research Funding: AstraZeneca, BeiGene, Novartis.
Open Research
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.





