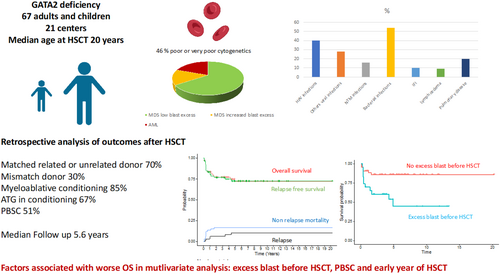
Long-term outcome after allogeneic stem cell transplantation for GATA2 deficiency: An analysis of 67 adults and children from France and Belgium
Marlene Pasquet and Regis Peffault de Latour contributed equally.
Summary
Modalities and timing of haematopoietic stem cell transplant (HSCT) in patients with GATA2 deficiency are still subject to debate. On June 2022, 67 patients (median age 20.6 years) underwent a first allogeneic HSCT among 21 centres. Indications for HSCT were myelodysplastic syndrome (MDS) ≤5% blasts ± immunodeficiency (66%), MDS >5% blasts (15%), acute myeloid leukaemia (19%). Conditioning regimen was myeloablative in 85% and anti-thymocyte globulins were used in 67%. The cumulative incidence (CInc) of acute graft versus host disease (GvHD) grade II–IV and III–IV at day 100 were 42% and 13%, and CInc of chronic and extensive chronic GvHD at 2 years were 42% and 23%. CInc of relapses was 3% and 11% at 1 and 5 years. Overall survival (OS) at 1 and 5 years was 83% and 72% (median follow-up 5.6 years). The factors associated with worse OS in multivariable analysis were the year of HSCT, a history of excess blasts before transplant and peripheral blood stem cell (PBSC) grafts. Age at HSCT, non-myeloablative conditioning and PBSC grafts were associated with increased non-relapse mortality. In conclusion, bone marrow monitoring to identify clonal evolution and perform HSCT before the appearance of excess blast is mandatory.
Graphical Abstract
The overall survival of patients receiving an allogeneic haematopoietic stem cell transplant (HSCT) for GATA2 deficiency was significantly better if they were transplanted recently, with a bone marrow or cord blood graft and if the transplant was performed before the onset of excess blast. Excess blast before HSCT was the only factor associated with an increased risk of relapse (independently of the cytogenetic and achieving remission before HSCT). Older age, non-myeloablative conditioning and peripheral blood stem cell graft were associated with an increased non-relapse mortality. Cumulative incidence of acute and chronic graft versus host disease (GvHD) observed in this study justifies the evaluation of different GvHD prophylaxis strategies. Bone marrow monitoring to identify clonal evolution and perform HSCT before the appearance of excess blast is mandatory.
INTRODUCTION
GATA2 deficiency is a protean congenital disorder associating haematological disorders, immunodeficiency (ID), pulmonary manifestations and various congenital abnormalities.1 Since 2011, progress has been made in understanding its pathophysiology and by defining the spectrum and characteristics of haematological, pulmonary and immunological manifestations. In particular, the cumulative incidence (CInc) of haematological malignancies has been assessed at 81% by the age of 402 but among myelodysplastic syndrome (MDS), different risk profiles emerged, with low-risk MDS appearing to be closer to bone marrow failure, in comparison with high-risk MDS/acute myeloblastic leukaemia (AML).3 Allogeneic haematopoietic stem cell transplantation (HSCT) remains the only curative therapy for haematological manifestations as well as for ID manifestations (human papilloma virus (HPV) or non-tuberculosis mycobacterial (NTM) infections) and has been shown to enable remission of some pulmonary diseases.4, 5 The first retrospective cohort of 21 HSCT recipients in 2014 reported a 4-year overall survival (OS) of 54%.1 At the same time, Grossman et al.6 reported disappointing outcomes after a non-myeloablative (NMA) conditioning regimen in a series of 14 patients mainly with low-risk haematological disorders and ID, with only eight patients alive at 3 years and two failure-related deaths. Children transplanted for MDS after a myeloablative conditioning (MAC) regimen was reported to have similar outcomes to non-GATA2 children in two large retrospective studies.7, 8 More recently, the updated cohort from the NIH including more patients transplanted with a haploidentical donor and the use of post-transplant cyclophosphamide (PTCy) in matched related (MRD) and unrelated donors (MUD) reported improved outcomes, with OS reaching 86% at 2 and 4 years.9 In MRD or MUD transplants, PTCy graft versus host disease (GvHD) prophylaxis appeared to yield better results than tacrolimus/methotrexate (MTX) prophylaxis, due to a very low rate of acute and chronic GvHD.9
Comparing these studies is difficult due to differences in terms of patient age, disease characteristics (notably, the proportion of high-risk myeloid malignancies), conditioning regimens and GvHD prophylaxis. In particular, in the latest report from the NIH,9 the proportion of patients with high-risk myeloid malignancies was surprisingly low. Moreover, the best timing and modalities of HSCT in each category of patients remain unclear.
In this paper, we retrospectively reviewed the medical records of patients with GATA2 deficiency transplanted in 21 French and Belgium centres until June 2022, firstly to assess the outcomes of patients with GATA2 deficiency after HSCT and secondly to look for prognostic profiles related to the disease spectrum.
METHODS
Study design, inclusion criteria and consent
We conducted a retrospective multicentric cohort study on behalf of the French Society for Bone Marrow Transplantation (SFGM-TC). All data were obtained in agreement with the Declaration of Helsinki from the Promise database and the French National Reference Centre for Chronic Severe Neutropenia Registry (CNIL—certificate No. 97.075). The study was approved by the scientific board of the SFGM-TC.
All patients with an inherited pathogenic variant of GATA2, who received an allogeneic HSCT before 10 June 2022, were included if they had provided an informed agreement. Patients with incomplete information about pretransplant disease and HSCT modalities, and who did not have at least 1 year of follow-up, unless deceased, were not included.
The following data were extracted from the medical records: (i) baseline data including sex, genetics, age at first symptoms, haematological data such as complete blood count results, bone marrow (BM) examination and cytogenetical exams; (ii) clinical data including infections (only serious viral infections were considered including opportunistic infections (such as CMV pneumonia), infections requiring hospitalization and those responsible for organ damage (such as extensive HPV disease)), thrombosis, lung and cutaneous diseases and congenital malformations, treatments received before HSCT; (iii) donor and recipient characteristics and HSCT modalities (in case of a second transplant, only the first was included); and (iv) follow-up and outcomes after HSCT. Sequential NMA conditioning regimen was defined as the sequential use of intensive chemotherapy and NMA conditioning regimen. Neutrophils recovery was defined as neutrophils count >0.5 G/L during 3 days and platelet recovery as platelet count >20 G/L during 3 days without transfusion the prior 5 days. Malignancies were assessed using the latest WHO classification10 (MDS with low blast count, ≤5% (MDS-LB), and increased blast counts, >5% (MDS-IB)), and R-IPSS was calculated using the MDS foundation calculator11; excess blasts before HSCT was defined as any history of MDS-IB or AML before HSCT, independently of the BM blast count at HSCT. CMV high risk was defined as a seropositive recipient transplanted with a seronegative donor or cord blood unit(s).
Study end-points
The primary end-point was OS. Secondary end-points were time to platelet and neutrophil recovery, graft failure, acute and chronic GvHD, relapse, non-relapse mortality (NRM), relapse-free survival (RFS)—including relapse or death, whichever occurred first, infections, GATA2 manifestations, all measured from time of HSCT. Grading of acute and chronic GvHD was performed as previously described,12 infectious complications were defined according to the ECIL criteria.13-15
Statistical analysis
Summary statistics, namely percentages for qualitative and binary variables and median (with interquartile range, IQR) for quantitative variables, are reported.
All failure time data were computed at the reference date of 10 June 2023. OS and RFS were estimated using the Kaplan–Meier method, with baseline comparisons based on the log-rank test. CInc of GvHD, relapse, NRM and infections were computed in a competing risks setting where death prior to the event of interest were treated as competing events, with comparisons based on the Gray test.
Prognostic analyses of OS and RFS used Cox model models with effect size measured on hazards ratio (HR), while those for competing end-points used Fine and Gray models, with effect size measured on subdistribution hazards ratio (sHR). Univariable then multivariable models were fitted, with model selection based on the Akaike criterion.
Point estimates and 95% confidence intervals (95% CI) were reported, with two-sided p-values.
All analyses were performed using R studio Version 1.2.1335.10
RESULTS
Sixty-seven patients (33 females, 49%) from 53 families were transplanted in 21 centres from 23 July 1989 to 9 June 2022, including 56 (84%) after 2010.
Pre-transplant medical history
Median age at first symptoms was 17.4 years (IQR, 12.7–24.4). Before HSCT, 36 (54%) had experienced serious viral infections, 36 (54%) at least one severe bacterial infection, 11 (16%) an NTM infection and 7 (10%) an invasive fungal infection. Pulmonary disease was reported in 20 patients including pulmonary alveolar proteinosis (PAP) in 5 (Tables 1 and 2).
| N = 67 | |
|---|---|
| Female (%) | 33 (49%) |
| Median age at first symptoms (years) [IQR] | 17.4 [12.7; 24.4] |
| Family history | 29 (43%) |
| At diagnosis | |
| Cytopenia | 20 (30%) |
| Cytopenia & immunodeficiency | 4 (6%) |
| MDS-LB | 22 (33%) |
| MDS-LB & immunodeficiency | 4 (6%) |
| MDS-IB | 5 (7%) |
| AML | 7 (10%) |
| Isolated immunodeficiency | 6 (9%) |
| History of infections | |
| All serious viral infections | 36 (54%) |
| HPV | 27 (40%) |
| Others serious viral infections | 19 (28%) |
| Non-tuberculous mycobacterial infection | 11 (16%) |
| Bacterial infections | 36 (54%) |
| Invasive fungal infection | 7 (10%) |
| Other manifestations of GATA2 deficiency | |
| Lymphoedema | 6 (9%) |
| Urogenital malformation | 8 (12%) |
| Pulmonary disease | 20 (30%) |
| Alveolar proteinosis | 5 (7.5%) |
| Bronchial dilatations | 3 (4.5%) |
| Emphysema | 4 (6%) |
| Bronchiolitis | 2 (3%) |
| Alveolar condensations | 5 (7.5%) |
| Thrombosis | 4 (6%) |
| Vascular malformations | 2 (3%) |
| Other malformations | 3 (4.5%) |
| Erythema nodosum | 4 (6%) |
| Aphtosis | 2 (3%) |
- Abbreviations: AML, acute myeloid leukaemia; HPV, human papilloma virus; HSCT, haematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MDS-IB, MDS increased blast; MDS-LB, MDS low blast.
| N = 67 | |
|---|---|
| Median time from first diagnosis to HSCT in years (y) [IQR] | 1.7 [0.8; 5.6] |
| Age at HSCT (y) [IQR] | 20.6 [14.5; 35.2] |
| ECOG at HSCT | |
| 0 | 38 (57%) |
| 1 | 26 (39%) |
| 2 | 3 (4%) |
| Haematological status at HSCT | |
| MDS-LB | 44 (66%) |
| MDS-IB | 10 (15%) |
| AML | 13 (19%) |
| ID involved in indication of HSCT | 7 (10%) |
| Active infection at HSCTa | 11 (16%) |
| History of excess blasts before HSCT | 23 (34%) |
| Cytogenetic abnormalities | |
| Poor or very poor cytogenetic | 31 (46%) |
| Monosomy 7 or del7 | 29 (43%) |
| Trisomy 8 | 15 (22%) |
| Der(1, 7) | 5 (7%) |
| Normal | 19 (28%) |
| R-IPSS | |
| Evaluable | 54 |
| Very low | 6 (11%) |
| Low | 22 (40%) |
| Intermediate | 14 (26%) |
| High | 10 (18%) |
| Very high | 2 (4%) |
| Treatment for myeloid malignancy before HSCTb | 13 (19%) |
| Complete remission post-targeted therapy at HSCT | 7 (54%) |
- Abbreviations: AML, acute myeloblastic leukaemia; HPV, Human Papilloma Virus; HSCT, Haematopoietic stem cell transplantation; ID, immunodeficiency; IQR, interquartile range; MDS-IB, myelodysplastic syndrome with increased blast excess; MDS-LB, myelodysplatic syndrome with low blast excess.
- a Non-tuberculous mycobacterial infections n = 5, bacterial infection n = 2 and Invasive fungal infections n = 3, active Hepatitis C infection n = 1.
- b Three patients received azacytidine before HSCT; none was in complete remission at HSCT (1 partial response); 11 received intensive chemotherapy before HSCT, 7 were in cytological CR at HSCT (including 1 with persistent cytogenetical abnormalities).
Recipient, donor and transplant characteristics at the time of HSCT
Median age at HSCT was 20.6 years (IQR, 14.5–35.2), and median time from first manifestations was 1.7 years (IQR, 0.8–5.6). Forty-four patients (66%) had MDS-LB, 10 (15%) MDS-IB and 13 (19%) AML. Cytogenetic was poor or very poor in 31 (46%) patients (partial or complete monosomy 7 in 29). Excluding patients with AML, the R-IPSS, available in 54 patients, was intermediate in 14 (26%) and high or very high in 12 (22%). Fourteen (21%) had received a treatment targeting myeloid malignancies, 7 (54%) of whom were in cytological remission (Tables 2 and 3).
| N = 67 | |
|---|---|
| Donor | |
| Matched related | 17 (25%) |
| Matched unrelated (10/10) | 30 (45%) |
| Mismatch unrelated (9/10) | 10 (15%) |
| Unrelated mismatch cord blood | 5 (7%) |
| Related haplo-identical | 5 (7%) |
| Donor female/recipient male | 13 (20%) |
| CMV high-risk | 12 (18%) |
| Conditioning regimen | |
| Myeloablative | 57 (85%) |
| TBI ≥ 8 Gy | 10 (23%) |
| UCY-based | 21 (37%) |
| BUFLU-based | 20 (35%) |
| MEL-based | 1 |
| Thiotepa BU-based | 5 (7%) |
| Sequential NMA | 5 (7%) |
| NMA | 5 (9%) |
| Source of cells | |
| BM | 28 (42%) |
| PBSC | 34 (51%) |
| CBU | 5 (7%) |
| GvHD prophylaxis | |
| ATG in conditioning | 45 (67%) |
| CsA & MTX | 32 (48%) |
| CsA & MMF | 15 (22%) |
| CsA | 10 (15%) |
| PTCy, CsA & MMF | 7 (10%) |
| αβ T cell depletion, CsA & MMF | 2 (3%) |
| CsA & prednisolone 1 mg/kg | 1 (2%) |
- Abbreviations: ATG, anti-thymocytes globulins; BM, bone marrow; BU, busulfan; BUCY, busulfan and cyclophosphamide; BUFLU, busulfan and fludarabine; CBU, cord blood unit; CMV, cytomegalovirus; CsA, ciclosporin; GvHD, graft versus host disease; MEL, melphalan; MMF, mycophenolate mofetil; MTX, methotrexate; NMA, non-myeloablative conditioning; PBSC, peripheral blood stem cell; PTCY, post-transplant cyclophosphamide; TBI, total body irradiation.
Donors were MRD in 17 (25%) patients, MUD in 30 (45%), mismatch-unrelated in 10 (15%), related haploidentical in 5 (7%) and unrelated cord blood unit(s) in 5 (7%). None of the related donors were reported to have GATA2 deficiency for patients transplanted before the genetical diagnosis. Conditioning regimens were myeloablative in 57 (85%) patients, NMA in 6 and sequential NMA in 5. Anti-thymocytes globulins (ATG) was used in 45 (67%) patients.
Outcomes
Median follow-up was 5.6 years (IQR, 3.4–8.2). Four patients were lost to follow up, at 1.0, 3.3, 4.6 and 9.1 years after HSCT respectively (Table 4).
| N (%) or median (IQR) | |
|---|---|
| Follow-up, months | 67 (40–99) |
| Engraftment | 66 (99%) |
| Secondary graft failure | 5 (7.5%) |
| Time from transplant, days IQR [;] (min, max) | 77 IQR [20;112] (20;383) |
| Early infections after HSCT (<6 months) | |
| All viral infections | 39 (58.2%) |
| CMV | 14 (20.9%) |
| EBV | 14 (20.9%) |
| BKV | 16 (23.9%) |
| Invasive fungal infection | 11 (16.4%) |
| Invasive aspergillosis | 7 (10.4%) |
| Other filamentous invasive infection | 2 (3%) |
| Candidiasis | 1 (1.5%) |
| Pneumocystis jirovecii infection | 1 (1.5%) |
| Disseminated toxoplasmosis | 1 (1.5%) |
| Other early complications | |
| Hemorrhagic cystitis | 20 (29.8%) |
| Lymphoedema worsening or appearance | 6 (9%) |
| Sinusoidal obstruction syndrome | 1 |
| Late complications (>6 months) | |
| Avascular osteonecrosis | 3 (4.5%) |
| Hypogammaglobulinemia requiring substitution | 2 (3%) |
| Pulmonary embolism | 1 (1.5%) |
| Pneumothorax | 1 (1.5%) |
| Relapse of hematologic malignancies | 6 (9%) |
| Median time from HSCT to relapse, days IQR [;] (min, max) | 383 [201;1028] (82,1597) |
- Abbreviations: BKV, BK virus; CMV, Cytomegalovirus; EBV, Epstein–Barr Virus; HSCT, Haematopoietic stem cell transplantation.
Engraftment was observed in all but one patient, with a median time to platelet and neutrophil recovery of 17 (IQR, 14–22) and 19 days (IQR, 15–21) respectively. The 63 evaluable patients at day 30 had complete blood donor chimerism.
A secondary graft failure was observed in five patients after a median time of 77 days (IQR, 20–112, range 20–383). Five of these six patients who experienced graft failures received a second transplant; three of them were alive 33, 40 and 114 months after the second transplant, respectively, while the other two died of relapse and GvHD, at 2 and 3 months after the second HSCT, respectively.
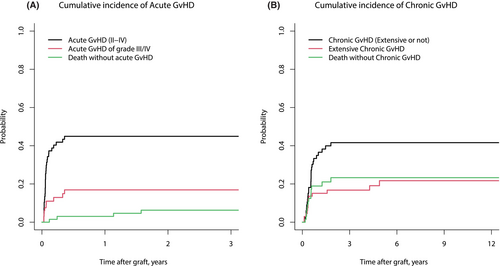
CInc of grade II–IV and grade III–IV acute GvHD at days 100 was 41.9% (95% CI, 29.9–53.4) and 12.9% (95% CI, 5.9–22.7) respectively (Figure 1A). CInc of chronic GvHD at 1 and 2 years was 34.9% (95% CI, 23.6–46.5) and 41.6% (95% CI, 29.4–53.4), respectively, and CInc of extensive chronic GvHD at 1 and 2 years were 18.9% (95% CI, 10.3–29.5) and 23.3% (95% CI, 13.3–34.9) respectively (Figure 1B). At the latest follow-up, 6 (12%) of the 50 patients who were alive still had immunosuppressive therapy.
No patient received post-transplant maintenance treatment. Six patients (5 AML and 1 MDS-LB) relapsed at a median of 374 days after HSCT (IQR, 201–1028; range 82–1597). CInc of relapse at 1 and 5 years was 3.0% (95% CI, 0.6–9.5) and 10.5% (95% CI, 4.2–20.3) respectively (Figure 2A). All died after relapse due to leukaemia progression, associated in one with an EBV-driven post-transplant lymphoproliferative disorder (PTLD).
CInc of viral infection at 1-year post-HSCT was 55.3% (95% CI, 42.5–66.4). Thirty-nine patients experienced a median of 1.5 infections (IQR 1–3): 14 experienced CMV infections (one CMV disease) and 13 required anti CMV therapy; 14 experienced EBV infections (2 had EBV PTLD) and 8 required rituximab therapy. Twenty (30%) patients experienced clinical haemorrhagic cystitis; BK virus infection (defined by a urinary BK viral load ≥7 log) was documented in 16 of them. Other infections were caused by HSV (n = 3), VZV (n = 3), enterovirus (n = 1), ADV (n = 1, requiring antiviral therapy), COVID-19 (n = 1) and HPV (n = 1). Eleven (16.4%) patients experienced invasive fungal infections (IFI) after HSCT mainly invasive aspergillosis (IA) (n = 7) (Table S1). Two of these seven patients had an history of IA before HSCT, all had favouring factors (mainly profound neutropenia or aGvHD) and all but one received mould chemoprophylaxis (caspofungin n = 4, voriconazole n = 1, posaconazole n = 1). Pre HSCT-diseases, donors and conditionings were various but 9 of the 11 received ATG.
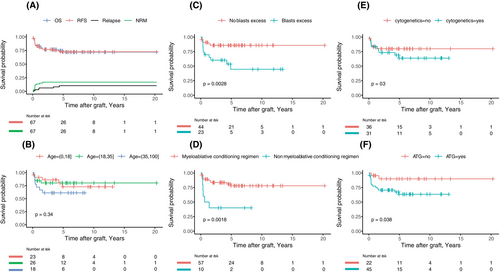
Seventeen patients died, with OS at 1, 2 and 5 years estimated at 83.4% (95% CI, 74.9–92.8), 76.8% (95% CI, 67.2–87.9) and 72.1% (95% CI, 61.3–84.7) respectively (Figure 2A). The causes of deaths were relapse in six patients, acute GVHD in six, infections in three, graft failure in one and chronic GVHD in one. Besides the six observed relapses, 11 patients died free of relapse, accounting for a total of 17 events, with 1-, 2- and 5-year RFS estimates at 83.4% (95% CI, 74.9–92.8), 76.8% (95% CI, 67.2–87.9) and 72.6% (95% CI, 62.1–84.8) respectively (Figure 2A).
Factors associated with outcomes
Among the 10 candidate predictors of the outcomes based on expert opinion, only those associated with an increased risk of death at the 10% level in univariable analyses (Table 5) were included in the multivariable models. Excess blasts before HSCT (HR 4.43, 95% CI, 1.58–12.39; p = 0.05), later year of transplant (HR 0.88, 95% CI, 0.81–0.96; p = 0.006) and non-PBSC graft (HR 0.3, 95% CI, 0.09–0.99; p = 0.049) were all selected by the Akaike criterion as adding to each other's prognostic information (Table S2). Survival probability according to these pretransplant parameters is illustrated in Figure 2. When the analysis was restricted to the 47 patients transplanted with an MRD (n = 17) or MUD (n = 30) (Tables S3 and S4), factors with significant impact on OS in multivariable analysis were excess blasts before HSCT (HR 5.53, 95% CI, 1.38–20.72; p = 0.0015) and an NMA conditioning regimen (HR 5.27, 95% CI, 1.4–19.8; p = 0.014). In this subset, OS at 1 and 5 years was estimated at 96.5% (95% CI, 90.1–100) and 89.6% (95% CI, 79.2–100) in the 30 patients transplanted without any history of BM excess blast, while they were 70.6% (95% CI, 51.9–95.9) and 46.8% (95% CI, 26.4–82.8) in the other subsets (p = 0.0029). OS was not significantly different between patients transplanted with MRD or MUD.
| Outcome | Overall survival, 17 deaths | RFS, 17 events | Relapse, 6 events | NRM, 11 events |
|---|---|---|---|---|
| HR (95%CI) | sHR (95%CI) | |||
| Year of HSCT | 0.94 (0.88–1.01) | 0.94 (0.88–1.01) | 0.91 (0.82–1.00) | 0.97 (0.90–1.05) |
| Time since diagnosis, /year | 0.99 (0.92–1.06) | 0.99 (0.92–1.06) | 1.03 (0.91–1.16) | 0.95 (0.87–1.04) |
| Age (/10 years) | 1.36 (0.98–1.87) | 1.35 (0.98–1.86) | 0.70 (0.23–2.12) | 1.61 (1.13–2.29) |
| <18 years | 0.76 (0.27–2.15) | 0.76 (0.27–2.15) | 1.84 (0.39–8.65) | 0.42 (0.09–2.00) |
| Excess blasts before HSCT | 4.07 (1.50–11.0) | 4.00 (1.48–10.9) | 10.2 (1.22–85.0) | 2.28 (0.71–7.33) |
| Cytogenetic poor/very poor | 1.65 (0.63–4.34) | 1.67 (0.64–4.40) | a | 0.62 (0.18–2.09) |
| Active infection at HSCT | 1.10 (0.31–3.81) | 1.12 (0.32–3.91) | 1.02 (0.12–8.31) | 1.14 (0.24–5.40) |
| Match related donor | 0.81 (0.26–2.48) | 0.81 (0.26–2.50) | 1.38 (0.25–7.60) | 0.60 (0.14–2.61) |
| NMA regimen | 4.33 (1.59–11.8) | 4.44 (1.62–12.2) | 3.27 (0.62–17.2) | 3.60 (1.10–11.8) |
| ATG in GvHD prophylaxis | 4.20 (0.96–18.4) | 4.16 (0.95–18.2) | 2.28 (0.27–19.3) | 5.23 (0.70–38.9) |
| Source of graft | ||||
| PBSC | 1.00 | 1.00 | 1.00 | 1.00 |
| BM or CBU | 0.37 (0.13–1.06) | 0.37 (0.13–1.05) | 2.08 (0.39–11.1) | 0.09 (0.01–0.76) |
- Note: All the 10 variables tested in the univariable analyses were selected based on prior expert knowledge regarding their potential association with the outcomes. Those actually associated with the outcome at the 10% level in univariable analyses are bolded, and were introduced into multivariable models. Significant effects at the 5% level are bolded.
- Abbreviations: ATG, anti-thymocyte globulin; BM, bone marrow; CBU, cord blood unit; HR, hazards ration; HSCT, hematopoietic stem cell transplantation; NMA, non myeloablative conditioning; NRM, non-relapse mortality; PBSC, peripheral blood stem cell; RFS, relapse-free survival; sHR, sub-distribution hazards ratio.
- a Convergence issues given all the 6 relapses occurred in patients with poor/very poor cytogenetics.
In the whole cohort, factors associated with CInc of NRM in univariable analyses were age at HSCT (sHR, 1.61, 95% CI, 1.13–2.3; p = 0.008), an NMA conditioning regimen (sHR, 3.66, 95% CI, 1.13–11.83; p = 0.031) and non-PBSC graft (sHR, 0.09, 95% CI, 0.01–0.76; p = 0.027). It should be noted that among patients transplanted with BM, no toxicity-related death was observed (4 patients died of malignancy relapse), while they were 10 in the PBSC group (2 relapse-related death) and 1 in the CBU group (no relapse-related death). By contrast, excess blasts before transplant was the only factor associated with the sHR (sHR, 0.09, 95% CI, 0.01–0.76; p = 0.027). The hazard of relapse did not differ between patients who achieved blast clearance before HSCT and those who did not (with or without treatment, data not shown).
Outcome of pretransplant HPV and pulmonary manifestations
Among the 27 patients with HPV manifestations at HSCT, 26 were evaluable: 25 (96%) experienced improvement and eventually complete remission, while a severe worsening of condylomas was observed in the latter requiring surgical resection (concomitant with AML relapse). In one patient, condyloma appeared during the first year after HSCT requiring local treatment.
Among the five patients with PAP, two are alive with complete resolution of pulmonary disease after HSCT; the three others died, one of respiratory failure in the context of graft failure 1 month after transplant, one of severe bronchiolitis obliterans 20 months after HSCT and one of refractory acute GVHD 4 months after HSCT.
Bronchiolitis (n = 2) and alveolar condensation (n = 5) disappeared after HSCT. In two of the four patients with emphysema, it worsened but both kept on smoking after HSCT.
None of the four patients with erythema nodosum has reported recurrence after transplant.
DISCUSSION
We report here the long-term outcomes of children and adult HSCT recipients with GATA2 deficiency, based on a French and Belgium retrospective multicentre cohort.
With a high prevalence of high-risk myeloid malignancies (30% of enrolled patients experienced AML or MDS-IB before HSCT, and 46% had poor or very poor cytogenetics according to R-IPSS criteria), frequent ID-related diseases (16% had NTM infections and 40% had HPV disease before HSCT), our cohort is representative of the GATA2 deficiency syndrome. HSCT modalities were based on the use of MAC regimens (85%) and ATG (67%) as GvHD prophylaxis.
Overall and event-free survivals were nearly similar, with estimated 1- and 2-year OS of 83.4% (95% CI, 74.9–92.8) and 76.8% (95% CI, 67.2–87.9). A history of excess blasts before HSCT, an early year of HSCT and a PBSC graft were independently associated with a decrease in survival in multivariable analysis. While OS and RFS were almost near similar, the time-dependent model identifies slightly different factors associated with RFS in multivariable analysis: year of HSCT, excess blasts before HSCT, NMA conditioning regimen and ATG used. This discrepancy highlights the link between some of these factors: Most of the children were transplanted with an MAC and a BM graft while ATG was more frequently used with PBSC and NMA regimen.
In patients transplanted without any history of excess blasts, 5-year OS was 85.7% (95% CI, 75.8–97.0) vs. 44.6% (95% CI, 26.0–76.8) in the others (p = 0.0028), highlighting the importance of early transplant. While age at transplant was unexpectedly not associated with OS in multivariable analysis, it was significantly associated with NRM. Finally, the year of HSCT was significantly associated with OS. While this could be due to the improvement of HSCT procedures, notably IFI prophylaxis, it also could illustrate the benefit of a disease-adapted strategy.
In 2022, Nichols-Vinueza et al.9 reported an excellent outcome after MRD and MUD HSCT with PTCy in comparison with MTX/tacrolimus-based GvHD prophylaxis (without ATG). Among their 59 recipients, only 4 (7%) had advanced myeloid malignancies and 10% had poor cytogenetic (monosomy 7), while 25% and 32% had an history of NTM and HPV respectively. These discrepancies do not allow straightforward comparisons between the two studies. Considering the relapse risk profile of our cohort, OS does not seem to get worse in comparison with their results. In the group of patients transplanted with a haploidentical donor and PTCY, the use of an NMA regimen as proposed by this team raised question in patients with high-risk myeloid malignancies, owing to the observed risk of relapse. AML and MDS IB relapse rates were high, suggesting that a maintenance therapy after HSCT should be proposed to these patients.
Regarding secondary end-points, engraftment was high (98%), but secondary graft failure occurred in 7.5% of the patients. This compares favourably to the graft failure rate of 15% with the NMA regimen proposed by Grossman et al.6 The use of BM as a graft source could have favoured graft failure (4/6 patients); however, NRM seems to be lower in patients transplanted with BM in our study as well as in Nichols-Vinueza's study.9
CInc of acute grade III–IV and extensive chronic GVHD were in the classical range8, 16 while higher than those observed with PTCy.9 The impact of GvHD on NRM in the context of pre-existing immunodeficiency with a high risk of viral and fungal complications is expected to be higher: This is an additional argument to promote the use of BM and to evaluate other GvHD prophylaxis such as PTCy. While ATG was not associated with worse OS in multivariable analysis, it was associated with worse RFS. However, in most cases, ATG was administered late in the conditioning period, without individual dose adjustment.17 A different use of ATG could help to limit the risk of graft failure, relapse and improve immune reconstitution.
CInc of viral infections was not unusual, excepted for symptomatic BK virus infections (observed in one-third of the patients independently of cyclophosphamide use). The frequency of invasive fungal infections, notably IA, highlights the importance of providing large spectra fungal prophylaxis from the start of conditioning.
The outcome of HPV infections was favourable in most of the patients: While one experienced a severe worsening of condyloma and one developed condyloma after HSCT, squamous cell carcinoma was not reported in our cohort, in contrast with a previous report.18 PAP disappeared in two of the five affected patients; the three others affected patients died, including two from respiratory distress. While the direct link between PAP and cause of death could not be established, this raised concern about the risk of pulmonary complications in those patients. This in line with the severe outcome observed by Marciano et al. who reported a crude mortality rate of 54% in patients with PAP.5 The duration of PAP before HSCT and the appearance of fibrosing lesion may explain the negative impact in some patients.
One of the main challenges of HSCT in patients with GATA2 deficiency is the timing of HSCT: Our study provides important clues regarding the best time point to perform transplant. A history of excess blasts before HSCT was the main prognostic factor of OS; in contrast, poor and very poor risk cytogenetics were not selected as independent factors associated with OS after HSCT. This highlights the importance of offering HSCT before excess blasts occur whenever possible. We recently proposed a classification of BM disorders in GATA2 deficiency based on cytological, cytogenetical and molecular profiles of our entire cohort and defined specific stage profiles associated with the occurrence of high-risk myeloid malignancies.3 A prospective follow-up based on regular somatic NGS screening to detect early markers of clonal evolution (SETBP1, RUNX1 and RAS pathway alteration) and cytogenetics could be proposed, so as to perform HSCT before excess blasts occur.
In conclusion, prognosis after HSCT in patients with GATA2 deficiency is strongly related to a history of excess blasts before HSCT and the use of PBSC. NMA and the use of ATG were not significantly associated with OS in multivariate analysis, but there were significantly associated with EFS. According to our results, our group recommends to, first, reserve NMA to unfit patients; second, to evaluate PTCY instead of ATG; and third, to privilege BM as graft source. The outcome of PAP patients after HSCT require larger studies to evaluate real benefit and risk in this indication.
AUTHOR CONTRIBUTIONS
FSDF, MP, JD and RPL designed the study; FSDF collected the data; KD, MRR and SC performed the statistical analysis and FSDF wrote the original version of the manuscript. FSDF, FC, MF, XP, EF, AS, BN, VG, STh, AG, BL, AM, SNQ, STa, LV, JD, MP and RPL took care and enrolled the patients in the registries. FSDF, FC, MF, XP, EF, AS, BN, VG, STh, AG, BL, AM, SNQ, STa, LV, JD, MP and RPL have contributed to data acquisition. FSDF, MRR, SC, RPL and MP have designed the research, interpreted the data and contributed to manuscript draft. All authors have approved and revised the manuscript and provided their agreement to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
We thank the patients and healthcare professionals who participated in this study, especially Dr Marie Ouachée (Institut d'Hématologie et d'Oncologie Pédiatrique, Lyon) Pr Isabelle Pellier (CHU d'Angers), Pr Jerôme Cornillon (Institut de Cancérologie et d'Hématologie Universitaire, Saint Etienne), Pr Mohamad Mohty (Hôpital Saint Antoine, APHP), Dr Benedicte Bruno (CHRU de Lille), Dr Louis Terriou (CHRU de Lille), Dr Aurelie Ravinet (CHRU de Clermont Ferrand), Dr Fanny Rialland (CHU de Nantes) and Dr Stanislas Nimubona (Hôpital Pontchaillou CHU de Rennes).
FUNDING INFORMATION
The study was not sponsored.
CONFLICT OF INTEREST STATEMENT
NA.
ETHICS APPROVAL STATEMENT
The ethical committee of the SFGM-TC has validated this study.
PATIENT CONSENT STATEMENT
An informed consent for data use has been signed by all the patients or their guardians before transplant.
CLINICAL TRIAL REGISTRATION
The study has been registered in HDH with the number F20231208140348.
Open Research
DATA AVAILABILITY STATEMENT
NA.