Biallelic inactivation of the NF1 tumour suppressor gene in juvenile myelomonocytic leukaemia: Genetic evidence of driver function and implications for diagnostic workup
Summary
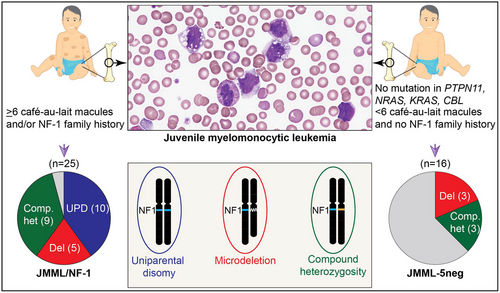
Juvenile myelomonocytic leukaemia (JMML) is characterized by gene variants that deregulate the RAS signalling pathway. Children with neurofibromatosis type 1 (NF-1) carry a defective NF1 allele in the germline and are predisposed to JMML, which presumably requires somatic inactivation of the NF1 wild-type allele. Here we examined the two-hit concept in leukaemic cells of 25 patients with JMML and NF-1. Ten patients with JMML/NF-1 exhibited a NF1 loss-of-function variant in combination with uniparental disomy of the 17q arm. Five had NF1 microdeletions combined with a pathogenic NF1 variant and nine carried two compound-heterozygous NF1 variants. We also examined 16 patients without clinical signs of NF-1 and no variation in the JMML-associated driver genes PTPN11, KRAS, NRAS or CBL (JMML-5neg) and identified eight patients with NF1 variants. Three patients had microdeletions combined with hemizygous NF1 variants, three had compound-heterozygous NF1 variants and two had heterozygous NF1 variants. In addition, we found a high incidence of secondary ASXL1 and/or SETBP1 variants in both groups. We conclude that the clinical diagnosis of JMML/NF-1 reliably indicates a NF1-driven JMML subtype, and that careful NF1 analysis should be included in the genetic workup of JMML even in the absence of clinical evidence of NF-1.
Graphical Abstract
Children with neurofibromatosis type 1 (NF-1) carry a defective NF1 allele in the germline and are predisposed to JMML, which presumably requires somatic inactivation of the NF1 wild-type allele. We examined the two-hit concept in leukaemic cells of 25 patients with JMML and clinical diagnosis of NF-1 and confirmed biallelic NF1 inactivation in all cases but one. Among 16 JMML patients without clinical signs of NF-1 and no variation in other JMML-associated driver genes, eight patients exhibited NF1-inactivating variants (six biallelic, two monoallelic). The data show that the clinical diagnosis of JMML/NF-1 reliably indicates a NF1-driven JMML subtype, and that NF1 genetic analysis should be included in the diagnostic workup of JMML even in the absence of clinical evidence of NF-1.
INTRODUCTION
Juvenile myelomonocytic leukaemia (JMML) is an aggressive clonal myeloproliferative and myelodysplastic neoplasia in children. Malignant transformation of haematopoietic stem/progenitor cells (HSPCs) into JMML is characterized by gene variants that promote sustained activation of the RAS signal transduction pathway. Variation of the canonical RAS pathway genes PTPN11, NRAS, KRAS, NF1 or CBL accounts for 90% of patients diagnosed with JMML.1 The majority of patients have no known predisposing condition and the RAS pathway variants (usually in PTPN11, NRAS or KRAS) occur somatically in HSPCs. Patients with Noonan syndrome and a germline variant in the PTPN11 (or, rarely, KRAS) gene are at increased risk of developing a JMML-like myeloproliferative disorder (MPD).2 Further predisposition to JMML includes CBL syndrome and neurofibromatosis type 1 (NF-1).3, 4 NF-1 is a complex multisystem developmental and tumour-predisposing disorder, which primarily affects the nervous system and skin,5 and has a prevalence between 1 and 10 cases per 10 000 children.6, 7 It has been estimated that patients with NF-1 are at approximately 200-fold risk of developing JMML compared to the general population.4, 8
Individuals with NF-1 typically carry one defective NF1 gene allele in the germline, with nearly half of the patients having de novo variants.9 NF1 is located near the centromere on chromosome band 17q11.2 in the vicinity of several repetitive elements, increasing its susceptibility to genetic alteration. Somatic inactivation of the inherited wild-type allele in haematopoietic cells is associated with transformation to JMML.4, 10 Biallelic NF1 inactivation was also reported in other tumours associated with NF-1, such as neurofibroma, malignant peripheral nerve sheath tumour and pilocytic astrocytoma, and was also found in hyperproliferative lesions, such as café-au-lait macules.11-14 Mitotic recombination, leading to uniparental isodisomy (UPD) of the 17q chromosome arm, was found to be the most frequent mechanism behind biallelic loss of NF1 function in JMML cells.15 Other mechanisms include somatically acquired deletions of 17q11.2 or inactivating variants on the second allele.16
Due to the presence of repeat sequences within the NF1 locus and the existence of several NF1 pseudogenes, it was historically difficult to obtain complete genetic analyses of the NF1 locus. Therefore, the diagnosis of the NF-1 group in patients with JMML was usually based on clinical features, chiefly café-au-lait macules, and/or a family history of NF-1. Taking advantage of advances in next-generation sequencing (NGS) technologies and better computational methods for the detection and interpretation of variants, we now set out to clarify whether the genetic findings in leukaemic cells of JMML/NF-1 patients are consistent with the clinical assessment and the two-hit concept. Specifically, the goal was to identify more than one NF1-inactivating event and thus confirm the relevance of clinical NF-1 features in children with JMML/NF-1. Furthermore, we investigated the possibility that the group of JMML patients without clinical evidence of neurofibromatosis and no abnormality in PTPN11, KRAS, NRAS or CBL (herein abbreviated as JMML-5neg) contained unrecognized cases driven by biallelic inactivation of NF1.
MATERIALS AND METHODS
The study cohort consisted of 156 patients diagnosed with JMML who were registered in the European Working Group of MDS in Childhood (EWOG-MDS) studies EWOG-MDS98 and EWOG-MDS2006 (NCT00047268 and NCT00662090; www.clinicaltrials.gov). Patients with Noonan syndrome-associated MPD or CBL syndrome were excluded by prior Sanger sequencing of PTPN11, KRAS, NRAS and CBL in hair follicles, buccal epithelium or skin fibroblasts. Parents or legal guardians of all patients provided informed consent to the scientific use of patient materials in accordance with the Declaration of Helsinki. The collection and storage of patient materials was approved by the institutional review board of each participating centre. DNA from bone marrow or peripheral blood cells collected at the time of diagnosis was used for targeted NGS. The multigene panel consisted of coding regions of canonical driver genes (PTPN11 [NM_002834], KRAS [NM_ 004985], NRAS [NM_002524], CBL [NM_005188, exons 7–10] and NF1 [NM_001042492]) and genes previously reported to be associated with JMML as secondary lesions (ASXL1 [NM_015338, exons 11–12], JAK2 [NM_004972.3, exon 14], JAK3 [NM_000215.3, exons 11–13,15,17,19], RRAS [NM_006270], RRAS2 [NM_012250], RAC2 [NM_ 002872], RUNX1 [NM_001754], SETBP1 [NM_015559, exon 4] and SH2B3 [NM_005475, exons 2–7]). The targeted NGS libraries were prepared using NEBNext Ultra II kits (New England Biolabs), and samples were sequenced on a MiSeq 2000 sequencer (Illumina) with 150 bp paired-end reads. CytoScan HD arrays (Affymetrix) were applied to detect segmental deletions or copy number-neutral loss of heterozygosity (LOH).
Our in-house bioinformatics pipeline was applied for the detection of sequence variants. The raw paired-end reads were trimmed using Trimmomatic (v0.30). Raw fastq files were processed with options 2:30:10, HEADCROP:3 TRAILING:10 MINLEN:25.17 The processed reads were mapped to the human genome (version hg19) using the BWA aligner (v 0.7.17) with mem mode.18 The aligned reads were further processed using Picard and GATK tools,19 converted into the mpileup format using Samtools v1.9 and subjected to variant discovery using VarScan v2.3.9.20, 21 We set the VAF cut-off as 5%, minimum coverage as 20 and p-value as 0.05. Subsequently, the variants were further filtered (--min-ref-basequal 28 --min-var-basequal 28 --min-ref-readpos 0.01 --min-ref-dist3 0.01 --min-var-readpos 0.01 --min-var-dist3 0.01) using VarScan2. The identified variants were annotated using Annovar, SnpEff and InterVar tools.22-24 We adhered to ACMG guidelines for annotating the variants.25 Variants were sequentially checked for their presence in the population databases gnomAD, ExAC, esp6500siv2 and 1000 Genomes. Variants with a population allele frequency above 0.1% were filtered out. Novel variants were manually checked against the alignment files to identify technical artefacts. NF1 is located near the centromere of 17q, is surrounded by repetitive elements and has several pseudogenes. We have identified and eliminated such off-target regions in our bioinformatics workflow to avoid the detection of spurious variants. Single-nucleotide variants and indels identified by our targeted NGS analysis and not previously listed in the ClinVar database were verified by Sanger sequencing.
RESULTS
Spectrum of NF1 gene alterations in patients with JMML
We examined the driver gene profile of 156 children with JMML by targeted NGS with a panel covering the full coding sequence of the NF1, PTPN11, KRAS, NRAS, RRAS and RRAS2 genes and the hotspot region (exons 7–10) of the CBL gene. Twenty-five of the 156 patients (16%) were clinically assigned to JMML/NF-1, based on the presence of ≥6 café-au-lait macules, or any number of café-au-lait macules plus positive family history. One hundred fifteen patients (74% of the 156 cases) exhibited somatic variants in the PTPN11, KRAS or NRAS genes and were negative for clinical features of NF-1. Sixteen children (10%) were JMML-5neg; that is, negative for clinical NF-1 and without genetic alteration of PTPN11, KRAS, NRAS or CBL in leukaemic cells. One of the 16 JMML-5neg patients carried a variant in RRAS2. Combining the JMML/NF-1 and JMML-5neg groups, the cohort for detailed genetic workup of NF1 thus consisted of 41 patients. These included 26 males and 15 females with a median age of 3.0 years at diagnosis (range, 0.2–7.8 years). The karyotype of leukaemic cells was normal in 31 cases, 5 cases had monosomy 7 and 5 carried other chromosomal abnormalities.
Among the 41 JMML cases subjected to in-depth NF1 molecular genetic analysis, we found 46 sequence alterations in 33 patients, consisting of 18 nonsense variants, 16 indels, 5 splice site variants, 5 missense variants and 2 in-frame deletions (Figure 1; Table S1). The variants were distributed across the entire NF1 sequence without obvious clusters or hot spots. p.I679Dfs*21, a single-nucleotide duplication in the homopolymer region (c. 2033dup), was identified in four patients. Two nonsense variants (p.R1362* and p.R1534*) were found in two patients each. All other variants occurred only once. Among 40 unique variants, 33 were predicted to cause premature truncation of the protein, in line with loss of neurofibromin function, the key mechanism of disease development. We also detected five missense variants in the cohort. These variants are not reported in the healthy population (gnomAD, v.2.1.1), affect amino acids with evolutionary conservation or 5′ splice sites of NF1 exons (Figure S1) and were in part reported in NF-1.26-28 All missense variants were predicted to impair protein function by several computational tools (Table S2).
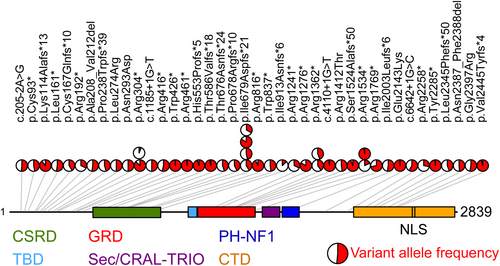
Two cases exhibited in-frame NF1 deletions entailing the loss of five and two amino acids respectively (p.A208_V212del and p.N2387_F2388del). Applying ACMG criteria,25 37/40 variants were pathogenic or likely pathogenic while 3/40 were scored as variants of unknown significance (Table S1). However, their non-random occurrence in concomitance with a pathogenic NF1 lesion supports their pathogenic role.
Additional NF1 alterations were detected by targeted NGS analysis in patients belonging to the JMML subgroups PTPN11 (9/76 cases, 12%) and NRAS (3/23 cases, 13%) (Table S3). No concomitant NF1 alterations were found in 16 patients of the KRAS subgroup, consistent with a previous exome analysis.29 The NF1 VAFs provided no evidence of biallelic NF1 inactivation in the JMML PTPN11 and NRAS subgroups, suggesting that these changes to NF1 were secondary events. The only exception was the case NRAS02 which harboured two pathogenic NF1 variants. Targeted sequencing in buccal epithelial cells demonstrated that the NRAS variant as well as both NF1 variants were absent from the germline. Single-cell DNA sequencing would be required to help determine whether NF1 or NRAS was the initiating oncogene in this case.
Genetic evidence of biallelic inactivation of NF1 in JMML
Upon evaluation of targeted NGS reads, 13 of the 25 JMML/NF-1 cases exhibited a NF1 loss-of-function variant with a variant allele frequency (VAF) in the range of 72%–99%, suggesting that these variants occurred in combination with a prior or later event causing LOH. Eight cases carried two independent pathogenic NF1 variants, each with a VAF of almost 50%, and one case harboured three NF1 variants with 53%, 28% and 13% VAF, suggesting two coexisting subclones. In the three remaining JMML/NF-1 cases, targeted sequencing identified pathogenic NF1 variants with monoallelic status (VAF 49%) in one sample and low VAF in the other two samples (32% and 6% respectively).
We then performed array-based single-nucleotide polymorphism (SNP) analysis to search for mechanisms of NF1 LOH in 15/25 JMML/NF-1 cases, that is excluding those nine cases where the combined occurrence of two or more heterozygous variants already suggested biallelic inactivation without LOH and one case where additional DNA for SNP arrays was unavailable. Among 15 cases, 10 exhibited a large region of UPD that affected almost the entire chromosome 17q arm and encompassed the NF1 locus (Table 1; Table S4), in line with previous reports.15, 16 Based on previous literature on 17q UPD-related neoplasms in NF-1,30 it is likely that the NF1 variation was the constitutional event, and subsequent somatic acquisition of 17q UPD led to JMML in these patients. We confirmed the acquired nature of 17q UPD in one case where sufficient remaining non-haematopoietic material (fibroblasts) was available (NF16 in Table 1). However, since the combination of a high-VAF variant and UPD is only possible if the UPD comes second, it can be assumed that all UPD cases had the isodisomy as the somatic event. In 3/15 cases, SNP array analysis detected microdeletions encompassing the NF1 locus (Table 1; Table S4). Of these, one was a recurrent type 1 deletion (i.e. encompassing 1.4 Mb and 14 protein-coding genes), one was a recurrent type 2 deletion (i.e. involving 1.2 Mb and 13 protein-coding genes but leaving the LRRC37B gene unaffected) and one was atypical.31 The remaining 2/15 cases showed NF1 variants with low VAF, suggesting that heterozygosity of NF1 was retained in leukaemic cells. However, SNP array analysis uncovered type 1 microdeletions in both (NF04 and NF12 in Table 1; Table S4). Non-haematopoietic material (fibroblasts) was available in three of the five microdeletion cases (NF09, NF12, NF13). The microdeletion was present but the missense variant was undetectable in all three germline samples, indicating that the sequence of constitutional microdeletion and acquired missense variant predominates in JMML/NF-1 cases without UPD. The possible combination of constitutional microdeletion and acquired 17q UPD was not detected in our cohort.
| ID | NF1 variant | NF1 amino-acid change | VAF (%) | Mechanism of NF1 LOH | Germline analysis | Secondary gene variants | Age (years) | Café-au-lait macules | Café-au-lait macule count | Other symptoms of NF-1 | Family history of NF-1 | Duration of follow-up (years) | Outcome | Previous reports |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NF01 | c.7035_7040delinsTA | p.(Leu2345Phefs*50) | 44 | None | Not done | None identified | 3.0 | Yes | 6 | No | No | 0.6 | Dead | |
| c.4600C>T | p.(Arg1534*) | 18 | ||||||||||||
| NF02 | c.4569del | p.(Ser1524Alafs*50) | 94 | UPD 17q | Not done | SETBP1 c.2602G>A, p.(Asp868Asn); 44.2% | 3.6 | Yes | ≥10 | Neurofibromas | Yes | 1.3 | Dead | |
| NF03 | c.5305C>T | p.(Arg1769*) | 72 | UPD 17q | Not done | None identified | 3.6 | Yes | ≥10 | Neurofibromas | No | 17.2 | Alive | Ref. 16 |
| NF04 | c.910C>T | p.(Arg304*) | 6 | Type 1 microdeletion | Not done | None identified | 0.5 | Yes | ≥10 | Neurofibromas | No | 19.5 | Alive | Ref. 16 |
| NF05 | c.6855C>A | p.(Tyr2285*) | 98 | UPD 17q | Not done |
ASXL1 c.1934dup, p.(Gly646Trpfs*12); 36.7% JAK3 c.1970G>A, p.(Arg657Gln); 48.0% |
5.2 | Yes | 6 | Neurofibromas | No information | 0.7 | Dead | Ref. 16 |
| NF06 | c.1277G>A | p.Trp426* | 99 | UPD 17q | Not done | ASXL1 c.1934dup, p.(Gly646Trpfs*12); 28.0% | 5.0 | Yes | ≥10 | Neurofibromas; freckling | Yes | 1.4 | Dead | Ref. 16 |
| NF07 | c.6642+1G>C | p.(Glu2214sp*)? | 58 | None | Not done | None identified | 0.6 | Yes | ≥10 | Neurofibromas | Yes | 15.7 | Alive | Ref. 16 |
| c.821T>G | p.(Leu274Arg) | 50 | ||||||||||||
| NF08 | c.1756_1759del | p.(Thr586Valfs*18) | 97 | UPD 17q | Not done | None identified | 1.4 | Yes | 8 | Neurofibromas | Yes | 0.5 | dead | Ref. 16 |
| NF09 | c.4110+1G>T | p.(Gln1370sp*)? | 98 | Type 1 microdeletion | Fibroblasts: Copy number loss chr17:28889920–30 501 044; absence of c.4110+1G>T |
ASXL1 c.1937dup, p.(Gly649Trpfs*9); 40.0% RUNX1 c.507_508+5dup, p.(?); 10.5% RUNX1 c.1090_1103del, p.(Ile364Valfs*231); 16.0% |
6.0 | Yes | ≥10 | Neurofibromas | Yes | 13.8 | Alive | Ref. 16 |
| NF10 | c.1657dup | p.(His553Profs*5) | 95 | Atypical 2.5 Mb deletion | Not done | JAK3 c.1969C>T, p.(Arg657Trp); 6.7% | 2.7 | Yes | ≥10 | No | Yes | 15.3 | Alive | |
| NF11 | c.6772C>T | p.(Arg2258*) | 28 | None | Not done |
ASXL1 c.1934dup, p.(Gly646Trpfs*12); 9.1% ASXL1 c.2077C>T, p.(Arg693*); 8.7% |
3.0 | Yes | ≥10 | No | No | 4.1 | Alive | |
| c.7189G>C | p.(Gly2397Arg) | 53 | ||||||||||||
| c.2737dup | p.(Ile913Asnfs*6) | 13 | ||||||||||||
| NF12 | c.574C>T | p.(Arg192*) | 32 | Type 1 microdeletion | Fibroblasts: Copy number loss chr17:29119495–30 228 792; absence of c.574C>T |
SETBP1 c.2608G>A, p.(Gly870Ser); 5.8% SETBP1 c.2602G>A, p.(Asp868Asn); 5.2% |
1.1 | Yes | ≥10 | No | No | 1.0 | Dead | |
| NF13 | c.910C>T | p.(Arg304*) | 87 | Type 2 microdeletion | Fibroblasts: Copy number loss chr17:29096017–30 498 238; absence of c.910C>T | CBL c.1139T>C, p.(Leu380Pro); 19.8% | 3.0 | Yes | ≥10 | No | No | 7.2 | Alive | |
| NF14 | c.4084C>T | p.(Arg1362*) | 44 | None | Not done | None identified | 4.3 | Yes | ≥10 | No | No | 8.4 | Alive | |
| c.279_280delinsAA | p.(Cys93*) | 47 | ||||||||||||
| NF15 | c.2033dup | p.(Ile679Aspfs*21) | 41 | None | Not done |
ASXL1 c.1934dup, p.(Gly646Trpfs*12); 35.0% SETBP1 c.2572G>A, p.(Glu858Lys); 27.0% |
1.8 | Yes | ≥10 | Optic glioma | No | 6.5 | Alive | |
| c.1185+1G>T | p.(Lys395sp*)? | 50 | ||||||||||||
| NF16 | c.2446C>T | p.(Arg816*) | 93 | UPD 17q | Fibroblasts: c.2446C>T not covered; UPD 17q absent | ASXL1 c.1934dup, p.(Gly646Trpfs*12); 38.0% | 3.6 | Yes | 7 | No | No information | 5.6 | Alive | |
| NF17 | c.1381C>T | p.(Arg461*) | 98 | UPD 17q | Not done | None identified | 2.0 | Yes | ≥10 | No | No | 2.1 | Dead | |
| NF18 | c.4235G>C | p.(Arg1412Thr) | 48 | None | Not done |
PTPN11 c.227A>C, p.(Glu76Ala); 10.7% ASXL1 c.2705dup, p.(Ser903Ilefs*3); 12.8% |
3.4 | Yes | ≥10 | No | Yes | 1.9 | Dead | |
| c.877A>G | p.(Asn293Asp) | 50 | ||||||||||||
| NF19 | c.7328_7331dup | p.(Val2445Tyrfs*4) | 96 | UPD 17q | Not done | SETBP1 c.2608G>A, p.(Gly870Ser); 14.9% | 3.0 | Yes | 4 | No | No information | 4.7 | Alive | |
| NF20 | c.7159_7164del | p.(Asn2387_Phe2388del) | 49 | None | Not done | CBL c.1246T>C, p.(Cys416Arg); 29.0% | 0.3 | Yes | 6 | No | No | 0.8 | Dead | |
| NF21 | c.4600C>T | p.(Arg1534*) | 98 | UPD 17q | Not done | ASXL1 c.2077C>T, p.(Gly693*); 43.6% | 3.1 | Yes | ≥10 | Neurofibromas | Yes | 13.3 | Dead | |
| NF22 | c.6427G>A | p.(Glu2143Lys) | 99 | UPD 17q | Not done | None identified | 1.7 | Yes | 1 | No | Yes | 18.8 | Alive | |
| NF23 | c.1246C>T | p.(Arg416*) | 54 | None | Not done | None identified | 1.4 | Yes | ≥10 | No | No | 15.0 | Alive | |
| c.2033del | p.(Pro678Argfs*10) | 43 | ||||||||||||
| NF24 | c.4084C>T | p.(Arg1362*) | 40 | None | Not done |
CBL c.1151G>A, p.(Cys384Tyr); 21.0% CBL c.1201_1203dup, p.(Cys401dup); 37.2% |
4.2 | Yes | ≥10 | Neurofibromas | No | 1.2 | Dead | Ref. 16 |
| c.205-2A>G | p.(Arg69sp*)? | 52 | ||||||||||||
| NF25 | c.499_502del | p.(Cys167Glnfs*10) | 41 | None | Not done | None identified | 3.0 | Yes | ≥10 | Neurofibromas | No | 14.5 | Alive | Ref. 16 |
| c.482T>G | p.(Leu161*) | 53 | ||||||||||||
| NEG01 | c.339dup | p.(Leu114Alafs*13) | 89 | Type 1 microdeletion | Buccal epithelium: c.339dup absent | None identified | 2.9 | No | No | No | 2.3 | Dead | ||
| NEG02 | c.711_723del | p.(Pro238Trpfs*39) | 46 | None | Fibroblasts: c.711_723del absent | ASXL1 c.2324T>A, p.(Leu775*); 28.6% | 2.6 | No | No | No | 13.4 | Alive | ||
| NEG03 | c.3721C>T | p.(Arg1241*) | 32 | Not assessed | Not done | ASXL1 c.1900_1922del, p.(649*); 36.0% | 5.6 | No | No | No | 13.4 | Alive | ||
| NEG04 | c.2033dup | p.(Ile679Aspfs*21) | 46 | None | Not done | None identified | 3.0 | No | No | No | 7.7 | Alive | ||
| c.6007-5A>G | p.(Ile2003Leufs*6) | 53 | ||||||||||||
| NEG05 | c.2024dup | p.(Thr676Asnfs*24) | 45 | None | Fibroblasts: c.2033dup and c.2024dup not covered | ASXL1 c.1934dup, p.(Gly646Trpfs*12); 32.0% | 2.6 | No | No | No | 13.1 | Alive | ||
| c.2033dup | p.(Ile679Aspfs*21) | 33/16.67 | ||||||||||||
| NEG06 | c.2033dup | p.(Ile679Aspfs*21) | 84 | Atypical 1.8 Mb deletion | Not done |
ASXL1 c.1774C>T, p.(Gln592*); 43.2% SETBP1 c.2602G>A, p.(Asp868Asn); 49.2% |
2.2 | No | No | No | 12.1 | Alive | ||
| NEG07 | c.3826C>T | p.(Arg1276*) | 83 | Type 1 microdeletion | Not done | None identified | 1.4 | No | No | No | 4.2 | Dead | ||
| NEG08 | c.2510G>A | p.(Trp837*) | 48 | None | Not done | None identified | 3.4 | No | No | No | 14.1 | Alive | ||
| c.622_636del | p.(Ala208_Val212del) | 52 | ||||||||||||
| NEG09 | No | No | No | Not assessed | Not done |
ASXL1 c.1934dup, p.(Gly646Trpfs*12); 5.6% RUNX1 c.514dup, p.(Ser172Lysfs*41);5% |
5.3 | No | No | No | 11.3 | Alive | ||
| NEG10 | No | No | No | Not assessed | Not done |
RRAS2 c.215A>T; p.(Glu72Leu);49% SETBP1 c.2572G>A; p.(Glu858Lys);46% JAK3 c.1970G>A, p.(Arg657Gln);44% |
5.9 | No | No | No | 6.3 | Alive | ||
| NEG11 | No | No | No | Not assessed | Not done | None identified | 0.5 | Yes | 2 | No | No | 13.0 | Alive | |
| NEG12 | No | No | No | Not assessed | Not done | None identified | 7.8 | No | No | No | 13.2 | Alive | ||
| NEG13 | No | No | No | Not assessed | Not done | None identified | 0.2 | No | No | No | 9.6 | Alive | ||
| NEG14 | No | No | No | Not assessed | Not done | None identified | 0.3 | No | No | No | 6.9 | Dead | ||
| NEG15 | No | No | No | Not assessed | Not done | None identified | 1.1 | No | No | No | 3.2 | Alive | ||
| NEG16 | No | No | No | Not assessed | Not done | None identified | 0.6 | No | No | No | 10.3 | Alive |
- Abbreviations: LOH, loss of heterozygosity; Mb, million base pairs; NF-1, neurofibromatosis type 1; UPD, uniparental disomy.
Among 16 patients in the JMML-5neg group, we identified three cases with NF1 variants at allelic frequency near 100% (NEG01, NEG06 and NEG07 in Table 1; Table S5) and three cases with compound-heterozygous NF1 variants (NEG04, NEG05 and NEG08), indicating biallelic loss of NF1 in the absence of syndromic features. SNP array analysis identified one atypical and two type 1 NF1 microdeletions in the three cases with high-VAF NF1 variants. One case carried two frameshift single-nucleotide duplications (c.2024dup and c.2033dup) in close proximity (NEG05). It was therefore possible to determine from individual sequencing reads that the two alterations were situated in trans, substantiating the concept of compound heterozygosity. Heterozygous or low-VAF NF1-inactivating variants, but no second hits to NF1, were found in two cases (NEG02 and NEG03), providing inconclusive evidence of driver function. There was no genetic evidence of NF1 involvement in the other eight JMML-5neg cases.
The lack of non-haematopoietic material in JMML-5neg cases with biallelic NF1 inactivation precluded us from assessing if any of the lesions were present in the germline. We could therefore not determine genetically whether the patients were affected by constitutional neurofibromatosis with the onset of JMML before the syndrome became clinically evident. However, the median follow-up period of 12.6 years without the children developing features of NF-1 (Table 1) argues against this idea. Other possible interpretations include double somatic NF1 hits in the haematopoietic lineage or postzygotic NF1 mosaicism.
Secondary variants associated with JMML and NF1 inactivation
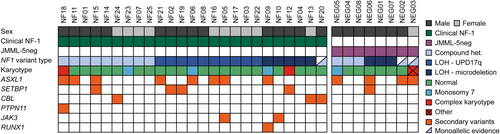
In addition to covering canonical RAS pathway driver genes, our NGS panel also interrogated secondary gene abnormalities frequently associated with JMML (i.e. those involving ASXL1, JAK2, JAK3, RUNX1, SH2B3 or SETBP1) and thus provided a picture of the variational landscape in NF1-driven JMML.29, 32-34 We found at least one secondary variant in 15 of the 25 patients with clinical NF-1, and in 4 of the 8 JMML-5neg patients with NF1 variants (Figure 2, Table 1). Among these, variants in the ASXL1 gene were present in 12 patients. All identified variants in ASXL1 lead to premature truncation due to a nonsense alteration, duplication of a single nucleotide or short deletion. Variants in the classic RAS pathway genes were found in four patients: The pathogenic PTPN11 variant p.E76A (VAF 11%) was found in one case, and the pathogenic CBL missense variants p.L380P, p.C384Y and p.C416R (VAFs ranging from 20% to 29%) were detected in three samples. The sample with the CBL p.C384Y variant additionally carried a three-nucleotide duplication in the CBL gene (VAF 37%), predicted to cause a non-frameshift insertion of one amino acid (p.C401dup). We identified at least one pathogenic SETBP1 variant (p.D868N, p.G870S, or p.E858K) in five patients, with VAFs ranging from 5% to 49%. The pathogenic JAK3 variants p.R657Q (VAF 48%) and p.R657W (VAF 7%) were found in one patient each. One sample harboured two RUNX1 variants (VAFs 11% and 16%). There was no significant difference between JMML/NF-1 and JMML-5neg patients with regard to secondary variants in genes that are not part of the canonical RAS pathway. However, all patients with secondary variants in RAS pathway genes (1 PTPN11 and 3 CBL) belonged to the JMML/NF-1 group.
DISCUSSION
The present study provides the first systematic investigation of genetic lesions that lead to biallelic loss of NF1 gene function and thus drive the leukaemic process in a large series of 41 children with JMML. A clinical diagnosis of NF-1 was known in 25 patients, while 16 patients had no clinical features of NF-1 and were also negative for variants in the other canonical RAS pathway driver genes PTPN11, KRAS, NRAS and CBL. Although we have previously addressed the genetics of NF1 variants or NF1 deletions in patients with JMML and NF-115, 16 and NF1 sequence analysis was part of previous whole-exome cohort studies,29, 32, 33 the additional value of the work presented here lies in a significantly increased number of cases, a more rigorous assessment of biallelic NF1 inactivation using improved sequencing methods and high-resolution SNP arrays, and a more detailed description of the neurofibromatosis phenotype. For clarity, patients already included in previous publications are indicated in Table 1.
The combined application of NGS and SNP array analysis identified biallelic NF1 inactivation due to gene variation plus LOH in 13 of 25 patients with JMML/NF-1 and 3 of 16 JMML patients with no clinical diagnosis of NF-1. Ten patients exhibited large regions of interstitial UPD involving almost the entire 17q chromosome arm. Six patients had NF1 microdeletions (four recurrent and two atypical). An interesting observation is that 17q UPD was restricted to JMML/NF-1 patients but did not occur in JMML-5neg children, and that it was detected only in the context of NF1 single-nucleotide variants or indels but not other types of NF1 inactivation. As a possible interpretation, UPD on top of an interstitial deletion would lead to nullisomy of other genes in the region and this could protect the cells from transformation to leukaemia. We also uncovered a high proportion of NF1 microdeletions in the cohort. These were germline events in all cases where non-haematopoietic material was available for testing. The overrepresentation of constitutional deletions in JMML/NF-1 patients compared to the general NF-1 population35 may suggest that patients with microdeletions are at higher risk of JMML, consistent with the view that codeleted flanking genes are likely to act as phenotype modifiers.31 SUZ12 deletion might be a potential link as inactivation of polycomb repressive complex 2 has previously been shown to be associated with JMML.29
Some interesting aspects emerged from the analysis of secondary variants in NF1-driven JMML. Consistent with the notion that JMML/NF-1 is a subtype with aggressive presentation and poor prognosis, secondary variants were detectable at the time of diagnosis in the majority (15/25) of JMML/NF-1 patients. Co-occurrence of another RAS pathway lesion with NF1 inactivation was noted in 4 of these 15 patients (3 CBL, 1 PTPN11). Both the clinical phenotype and the comparison of allele frequencies clearly indicated that NF1 was the original driver in these cases whereas the changes in the other RAS pathway genes were limited to subclones. Further, the JMML/NF-1 group contained eight patients with ASXL1 lesions and four with SETBP1 alterations. Concerning ASXL1 in particular, this suggests an overrepresentation compared to patients with other subtypes of JMML.29, 32-34
For the management of patients, we conclude from our study that the clinical assignment to the JMML/NF-1 group is reliable and can almost always be confirmed genetically. This is remarkable since the children are often too young at the onset of JMML to display the full spectrum of NF-1-associated symptoms.5, 36 In fact, our genetic data show that the presence of ≥6 café-au-lait macules (or less in the case of affected parents) in a child with JMML is already sufficient to diagnose NF-1 with a very high probability.
Our data illustrate that the two definitions of the NF1-driven JMML subtype recently published by expert groups have imperfections.37, 38 The ICC definition calls for “germline NF1 mutation and LOH of NF1 or clinical diagnosis of NF-1”, but this does not accommodate compound-heterozygous variants in children with clinical NF-1 or double somatic NF1 inactivation in children without NF-1 phenotype.37 The WHO definition requires “biallelic pathogenic alterations in NF1” but this does not account for JMML cases with a clear clinical diagnosis of NF-1 where genetic NF1 analysis is uninformative or missing.38 Both definitions do not specify whether testing for LOH (e.g. by SNP array) is explicitly required or whether LOH can be inferred from a high NF1 VAF; also, no VAF thresholds are provided for the latter alternative.
Our genetic analysis in JMML patients who lacked clinical features of NF-1 and harboured no driver alteration in PTPN11, KRAS, NRAS or CBL demonstrated the biallelic involvement of NF1 in a relevant number of cases (38%). Since no NF-1 symptoms developed in these children even after long observation periods, we consider postzygotic mosaics or double somatic NF1 inactivation in haematopoietic cells to be more likely than constitutional NF-1. Future studies are needed to determine whether this variety of NF1-driven JMML differs haematologically and clinically from JMML in constitutional NF-1. Finally, in JMML-5neg cases without NF1 alteration, other forms of myeloproliferative neoplasms should be considered, for example, those involving alterations or rearrangements of tyrosine kinase genes.
AUTHOR CONTRIBUTIONS
Senthilkumar Ramamoorthy, Dirk Lebrecht, Denny Schanze, Ina Schanze, Ilse Wieland, Geoffroy Andrieux, Patrick Metzger, Maria Hess, Marcin W. Wlodarski, Melanie Boerries, Martin Zenker and Christian Flotho performed experiments, analysed the data and interpreted variants. Michael H. Albert, Arndt Borkhardt, Dorine Bresters, Jochen Buechner, Albert Catala, Valerie De Haas, Michael Dworzak, Miriam Erlacher, Henrik Hasle, Kirsi Jahnukainen, Franco Locatelli, Riccardo Masetti, Jan Stary, Dominik Turkiewicz, Luca Vinci, Ayami Yoshimi and Charlotte M. Niemeyer provided clinical samples, collected and interpreted clinical phenotypes, and analysed the data. Martin Zenker and Christian Flotho conceived the study. Senthilkumar Ramamoorthy and Christian Flotho wrote the manuscript. All authors revised the manuscript.
ACKNOWLEDGEMENTS
We are grateful to the patients who participated in the study and their families and referring physicians. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (grants CRC1160-Z02, CRC/TRR167-Z01, CRC1453-S1 and CRC1479-S1 to M.B., and CRC992-C05 to C.F.), Deutsche José Carreras Leukämie-Stiftung (DJCLS 15R/2022 to S.R.) and the German Federal Ministry of Education and Research (BMBF) (grants MyPred 01GM1911A to M.E., M.W.W., C.M.N. and C.F.; MIRACUM-FKZ 01ZZ1801B to M.B.; and EkoEstMed-FKZ 01ZZ2015 to G.A.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by Research Ethics Committee “Ethik-Kommission der Albert-Ludwigs-Universität”, University of Freiburg, Germany.
PATIENT CONSENT STATEMENT
The authors attest to the fact that written consent for participation was received from every individual whose data are included.
CLINICAL TRIAL REGISTRATION NUMBER
European Working Group of MDS in Childhood (EWOG-MDS) studies EWOG-MDS98 and EWOG-MDS2006 (NCT00047268 and NCT00662090; www.clinicaltrials.gov).