CD30 as a therapeutic target in adult haematological malignancies: Where are we now?
Summary
CD30 is a transmembrane protein from the tumour necrosis factor receptor superfamily. It is expressed on a small subset of activated T and B lymphocytes, and various lymphoid neoplasms. CD30 is a particularly interesting treatment target because its levels are high in tumours but low in healthy tissues. Several therapeutic strategies targeting CD30 have been developed, including monoclonal antibodies, conjugated antibodies (combination of brentuximab vedotin with chemotherapy or immunotherapy), bispecific antibodies and cell and gene therapies, such as anti-CD30 CAR-T cells in particular. We briefly review the biology of CD30 which makes it a good therapeutic target, and we describe all of the anti-CD30 therapies that have emerged to date.
CHARACTERIZATION AND EXPRESSION OF CD30
CD30 (CD = cluster of differentiation), also known as Ki-1 or TNFRSF8 (tumour necrosis factor receptor superfamily member 8), was first described in 1982, in Hodgkin and Reed-Sternberg cells from patients with Hodgkin lymphoma.1 CD30 is a 120 kDa type I transmembrane glycosylated protein composed of 595 amino-acid residues forming an intracellular domain, a transmembrane domain and an extracellular domain.2 The extracellular domain consists of six cysteine-rich regions in a duplicated structure (Figure 1).
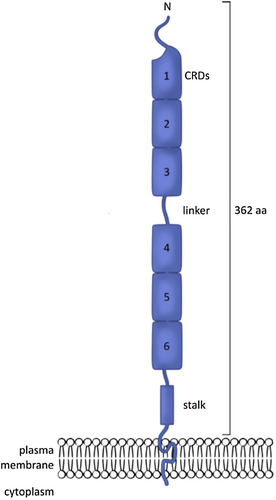
All TNF family proteins form homotrimers, a configuration essential for their functionality. In vitro, experiments have suggested that CD30 also has a homotrimeric structure, consistent with the symmetry required for the binding of this molecule to its ligand.3
Studies based on epitope mapping for several monoclonal antibodies and molecular modelling revealed that the CD30 homotrimer adopted a flower-like structure. A large number of epitopes and antibody-binding sites (e.g. Ki-1, Ki-2 binding domains) were found to be located in inflexible loops on the surface of the molecule easily accessible to antibodies.3
The extracellular part of the membrane-bound CD30 can be cleaved proteolytically by a zinc metalloprotease (TNF-α-converting enzyme = TACE, also known as ADAM17).4, 5 This enzyme cleaves the CD30 molecule in the juxtamembrane extracellular portion, and this cleavage can be blocked with TACE selective inhibitors.6 It generates a soluble form of CD30 (sCD30) with a molecular mass of 85 kDa.4
In healthy tissues, CD30 expression is restricted to a subset of activated T and B lymphocytes located around the B-cell centre follicles of the lymphoid tissues and, to a lesser extent, at the edge of germinal centres, but it is not usually found in the peripheral blood.4
Moreover, CD30 expression has been detected in various lymphoid malignancies, the highest levels of expression being recorded for classical HL and anaplastic large-cell lymphoma (ALCL). Variable expression and intensity have been observed in some cases of peripheral T-cell lymphoma, cutaneous T-cell lymphoma (CTCL), extranodal NK-T-cell lymphoma and in several B-cell non-Hodgkin lymphomas, including diffuse large B-cell lymphoma (DLBCL).5
Increases in sCD30 have been observed in patients with HL (with a median sCD30 level about 20 times higher than that in healthy controls7), ALCL (1500 times higher7), adult T-cell leukaemia and angioimmunoblastic T-cell lymphoma (AITL).4, 8-10 Serum sCD30 levels are generally correlated with tumour mass, and associated with advanced disease stage and poor prognosis.4 Moreover, sCD30 levels decrease in response to effective treatment and increase in case of relapse.4
CD30 has a pleiotropic biological function; it acts as a co-stimulation receptor.4 The natural ligand of CD30 is CD153 (CD30L), a glycoprotein of the TNF family expressed on activated T cells, macrophages, B cells, neutrophils, eosinophils and mast cells. The binding of CD30 to CD153 induces the activation, proliferation, differentiation and apoptosis of various cell types displaying activation and enhanced cytokine secretion in HL cells, but a pro-apoptotic effect in ALCL cell lines.11 Gene expression profiling has revealed a marked difference in gene transcription between HL and ALCL cell lines in terms of the response to antibody binding to CD30 in HL.12-14
Given its very low levels of expression in healthy tissues, CD30 has been identified as an attractive potential treatment target, and growing numbers of anti-CD30 therapeutic agents are being tested. In this review, we describe all the anti-CD30 therapies that have emerged to date (see Table 1 and Figure 2).
| Type of haematological malignancy | In vitro/in vivo | Type of therapy | Name of the therapy | Number of evaluable patients | Efficacy (%) | Safety (%) | Ref. |
|---|---|---|---|---|---|---|---|
| T-cell leukaemia cell lines ALCL murine xenograft model | In vitro | Monoclonal antibody | SGN-30 | NA | Cell growth arrest | 15-17 | |
| CD30+ haematological malignancies | In vivo | Monoclonal antibody | SGN-30 | 79 | 29% SD | 36.7% grade 3–4 AE | 18-20 |
| 24 |
4% CR 25% SD |
17% grade 3–4 AE | |||||
| 23 |
43% CR 26% PR |
13% grade 3–4 AE | |||||
| CD30-expressing cell lines | In vitro | Monoclonal antibody | MDX-060 | NA | Cell growth inhibition | 21 | |
| HL xenograft model in SCID mice | In vivo | Monoclonal antibody | MDX-060 | NA | C | 21 | |
| CD30+ lymphoma | In vivo | Monoclonal antibody | MDX-060 | 72 | 8% CR | 7% grade 3–4 treatment-related AE | 22 |
| SCID mice | In vitro In vivo | Antibody-drug conjugate | Ki-4.dg4 | NA | Tumour growth inhibition | 23 | |
| R/R HL | In vivo | Antibody-drug conjugate | Ki-4.dg4 | 15 |
6% PR 6% minor response 12% SD |
Vascular leak syndrome is Less well tolerated than other similar immunotoxins | 25 |
| HL and ALCL cell lines | In vitro | Antibody-drug conjugate | BV | NA | Cell cycle arrest and apoptosis induction 1 PR and 1 CR | 27 | |
| R/R DLBCL | In vivo | Antibody-drug conjugate | BV | 48 |
17% CR 13% PR ORR of 44% |
18% grade 4 neutropenia 10% treatment-related death |
29 |
| R/R DLBCL | In vivo | Antibody-drug conjugate Immunomodulator | BV Lenalidomide | 37 |
35% CR ORR of 57% |
Well-tolerated GCSF support for neutropenia | 30 |
| R/R PMBL after SCT and 1–2 prior therapies | In vivo | Antibody-drug conjugate PD1 inhibitor | BV Nivolumab | 30 |
37% CR ORR of 73% |
53% of grade 3–4 treatment-related AE | 33 |
| PEL cell lines | In vitro | Antibody-drug conjugate | BV | NA | Cell proliferation inhibition (G2/M arrest and apoptosis induction) | 36 | |
| R/R PEL after one or more lines or prior treatment | In vivo | Antibody-drug conjugate | BV |
4 (3 HIV+ and 1 HIV−) |
75% CR 25% PD |
37-39 | |
| R/R T-cell lymphoma | In vivo | Antibody-drug conjugate | BV | 128 |
17.2% CR ORR of 54.7% |
5% grade 3 peripheral neuropathy | 42 |
| R/R ALCL | In vivo | Antibody-drug conjugate | BV | 58 |
57% CR ORR of 86% |
60% grade 3–4 AE | 43 |
| First-line treatment of HL | In vivo | Antibody-drug conjugate Chemotherapy | BV AVD | 664 | 3-year PFS of 83.1% |
83% grade 3–5 AE (with 1% treatment-related deaths) |
45, 46 |
| First-line treatment of HL in elderly patients | In vivo | Antibody-drug conjugate PD1 inhibitor | BV Nivolumab | 46 | 48% CR |
11% grade 3 peripheral neuropathy 8% grade 4 AE 2% treatment-related deaths |
48 |
| R/R HL | In vivo | Antibody-drug conjugate | BV | 102 | 34% CR | 55% grade 3–4 AE | 31, 49 |
| R/R HL after SCT | In vivo | Antibody-drug conjugate | BV | 165 |
PFS of 42.9 months 5-year PFS of 59% |
56% peripheral neuropathy 35% neutropenia |
50, 51 |
| R/R HL | In vivo | Antibody-drug conjugate | BV | 153 | PFS of 8.3 months | 25% grade 3–4 AE | 52 |
| R/R HL | In vivo | Antibody-drug conjugate PD1 inhibitor | BV Nivolumab | 93 |
67% CR ORR of 85% |
18% irAE without treatment discontinuation 7% grade 3–4 pneumonitis, maculopapular rash, increased aspartate aminotransferase levels, diarrhoea and Guillain-Barré syndrome | 53 |
| 61 |
57% CR ORR of 76% |
16% grade 3–4 treatment-related AE 1.5% treatment-related death |
54 | ||||
| R/R HL | In vivo | Antibody-drug conjugate CTLA4 inhibitor | BV Ipilimumab |
61% CR ORR of 89% |
43% grade 3–4 treatment-related AE | 54 | |
| R/R HL | In vivo | Antibody-drug conjugate PD1 inhibitor CTLA4 inhibitor | BV Nivolumab Ipilimumab |
73% CR ORR of 82% |
50% grade 3–4 treatment-related AE 1.5% of treatment-related deaths |
54 | |
| SCID mice bearing CD30+ human HL | In vitro | Bispecific antibody | HRS-3/A9 | NA | NK cell recruitment and activation Complete remission | 55 | |
| R/R HL | In vivo | Bispecific antibody | HRS-3/A9 | 15 |
6% CR 6% PR |
60% anti-drug antibody development | 56-58 |
| Mice** | In vitro In vivo | Bispecific antibody | AFM-13 | NA | NK cell cytotoxicity enhancing the cytokine production of IL12/15/18-induced memory-like mature blood NK cells | 59 | |
| R/R HL | In vivo | Bispecific antibody | AFM-13 | 28 | 11.5% PR ORR of 23% (dose ≥1.5 mg/kg) | Mild-to-moderate AE 53.6% of patients developed anti-drug antibodies development (50% potentially neutralizing) | 60 |
| R/R HL after BV | In vivo | Bispecific antibody PD1 inhibitor | AFM-13 Pembrolizumab | 30 |
37% CR 47% PR ORR of 83% |
Well tolerated 3% treatment-related grade 3 AE | 61 |
| CD30+ HL cell lines | In vitro | 1st generation CAR-T cells | Anti-CD30 CAR-T cells | NA | 63, 64 | ||
| R/R HL | In vivo | 2nd generation CAR-T cells | Anti-CD30 CAR-T cells | 41 |
29% CR, ORR of 72% for patients with fludarabine-based lymphodepletion No OR for patients with bendamustine-based lymphodepletion |
24% grade 0–1 CRS No neurotoxicity 10% grade 3–4 AE (thrombocytopenia) | 65 |
| R/R HL | In vivo | 2nd generation CAR-T cells | Anti-CD30 CAR-T cells | 18 |
39% PR 33% SD |
11% of patients with grade 3–4 AE | 66 |
| T-cell lymphoma, mouse models | In vitro In vivo | 2nd generation CAR-T cells | Anti-CD30 CAR-T cells | NA | Cytotoxic effect, tumour inhibition | 67 | |
| CD30+ lymphoma | In vivo | 2nd generation CAR-T cells | Anti-CD30 CAR-T cells (106/kg) | 4 | 75% PR | 33% of patients with CRS have No neurotoxicity | 68 |
| 2nd generation CAR-T cells | anti-CD30 CAR-T cells (107/kg) | 3 |
66% CR 33% PR |
||||
| 2nd generation CAR-T cells PD1 inhibitor |
Anti-CD30 CAR-T cells (107/kg) ? |
5 |
80% CR ORR of 100% |
||||
| R/R HL | In vivo | 2nd generation CAR-T cells 2nd generation CAR-T cells | Anti-CD19 CAR-T cells Anti-CD30 CAR-T cells | 1 | CR | No severe toxicity | 69 |
- Abbreviations: AE, adverse events; ALCL, anaplastic large T-cell lymphoma; AITL, angioimmunoblastic T-cell lymphoma; BV, brentuximab vedotin; CAR, chimeric antigen receptor; CR, complete response; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; ORR, overall response rate; PFS, progression-free survival; PMBCL, primary mediastinal large B-cell lymphoma; PR, partial response; SAE, severe adverse event; SCT, stem-cell transplantation; SD, stable disease.
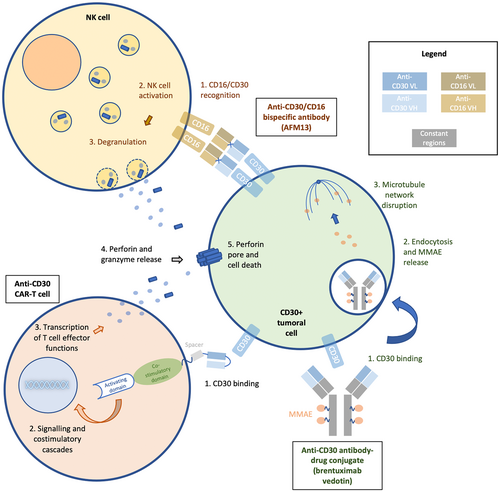
Schematic of 3 types of anti-CD30 therapies. This illustration is the property of the authors.
The bispecific antibody AFM13 simultaneously binds to the CD30 antigen and CD16A on NK cells. This leads to the formation of an “immunological synapse”, subsequent activation of NK cells and release of cytotoxic substances, granzyme B and perforin, finally leading to lysis of CD30-positive cells; Brentuximab vedotin acts by binding to CD30-positive cells, leading to receptor endocytosis and MMAE released on exposure to intracellular lysozymes, resulting in an inhibition of tubulin formation and cell apoptosis; After binding of the anti-CD30 CAR-T cell to his target, downstream signalling cascade and costimulatory signalling lead to T cell effector functions including the release of perforin and granzyme leading to CD30-positive cell death.
CD30 AS A TREATMENT TARGET
- Monoclonal antibody monotherapy
A “naked” monoclonal antibody (mAb), SGN-30 (a chimeric mouse-human antibody), gave very encouraging results in preclinical studies, in which it halted the growth of adult T-cell leukaemia cell lines, and in a murine xenograft model of human CD30+ ALCL.15-17 Clinical trials conducted at the start of the 2000s yielded disappointing results for various CD30+ haematological malignancies (with no partial or complete response), leading to the discontinuation of development for the use of this antibody in monotherapy.18-20
- Immunotoxins and antibody-drug conjugate (ADC)
Immunotoxins are chimeric proteins made of a highly toxic component derived from a natural toxin from which the “binding” portion has been replaced by a modified antibody or antibody fragment targeting a specific malignant cell type. In the late 1990s, an immunotoxin (Ki-4.dg4) constructed from the anti-CD30 mAb Ki-4 and a deglycosylated ricin A-chain (a potent ribosomal poison produced by seeds of the castor bean that inhibits tumour growth23, 24) yielded encouraging results in vitro and SCID mice.23 It was tested in phase I clinical trial, in patients with refractory Hodgkin lymphoma. Clinical responses were evaluable in 15 patients, one of whom presented partial remission (PR), one a minor response and two stable diseases (SD). Adverse effects and dose-limiting toxicities were related to vascular leak syndrome (16/17 patients): decreases in serum albumin concentration, oedema, weight gain, hypotension, tachycardia, myalgia and weakness.25 At an equivalent dose, the Ki-4.dg4 immunotoxin caused more grade 3–4 adverse events than other similar immunotoxins (e.g., the RFT5.dgA anti-CD25 immunotoxin). The moderate efficacy observed may be at least partly due to the formation of Ki-4.dgA/sCD30 complexes.26
ADCs are complex molecules composed of an antibody linked to a cancer-killing drug, combining the specific and cytotoxic properties of each component. Brentuximab vedotin (BV or SGN-35) is an ADC composed of the humanized IgG1 mAb SGN-30 conjugated to the antimitotic agent monomethylauristatin E (MMAE) via a cathepsin-cleavable linker. BV acts by binding to CD30-positive cells, leading to ADC endocytosis and MMAE release on exposure to intracellular lysozymes, resulting in an inhibition of tubulin formation and cell apoptosis (Figure 2).27 The development of this novel ADC appeared to overcome some of the problems seen with the “naked” monoclonal antibody, SGN-30.
Preclinical studies confirmed the efficacy of BV in vitro, in HL and ALCL cell lines in particular, and mouse models, demonstrating the stability of the ADC and its selectivity for CD30-positive cells. These studies also confirmed that BV was able to halt the cell cycle and induce apoptosis. BV treatment resulted in partial tumour regression in a mouse model of HL and complete tumour regression in a mouse model of ALCL, with tumour responses significantly different from those obtained with the “naked” antibody.27
Brentuximab vedotin in clinical trials
Clinical trials with BV led, for the first time an anti-CD30 therapy, to clinical practice change.
During clinical trials, the toxicity of the conjugate included, when combined with chemotherapy, febrile neutropenia for which patients subsequently received recommended primary prophylaxis with granulocyte colony-stimulating factor and peripheral neuropathy, without resolution in several patients.
Classical Hodgkin lymphoma (HL)
HL is the most common lymphoma of adolescents and young adults. About 80% of HL are cured by the standard first-line chemotherapy with doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD), but treating R/R HL patients remains challenging. Indeed, current second-line and subsequent therapies are of limited efficacy, with remission achieved in fewer than one-quarter of patients with R/R disease.
HL is certainly the haematological malignancy for which BV has been the most extensively studied, either alone or in combination, in first-line regimens and beyond.
First-line regimens
The randomized ECHELON-1 clinical trial compared the addition of BV to a doxorubicin, vinblastine and dacarbazine (AVD) regimen with standard first-line chemotherapy (ABVD, delivered fortnightly for up to six cycles of 28 days), in patients with stage III or IV HL. After a median follow-up of 24.6 months, PFS at 2 years was 82.1% (95% confidence interval [CI], 78.8%–85.0%) in the BV + AVD group and 77.2% in the ABVD group (95% CI, 73.7%–80.4%).28 After a median of 37 months of follow-up, PFS at 3 years was 83.1% (95% CI, 79.9%–85.9%) for BV + AVD and 76.0% (95% CI, 72.4%–79.2%) for ABVD. The overall survival was also longer in the BV + AVD group with 93.6% of patients still alive at 6 years (vs. 89.4% in the ABVD group).29 First-line treatment of stage III or IV HL with BV + AVD had sustained benefits over ABVD30 but was associated with greater toxicity.
The added value of adding BV to first-line treatment for patients with advanced HL seemed to be maintained in high-risk patients (stage IV), with a three-year PFS of 81.8% (95% CI, 77.6%–85.3%) for the BV + AVD group, versus 74.9% (95% CI, 70.2%–78.9%) for the ABVD group.31
For first-line treatment in the elderly (or chemotherapy-ineligible) population, the combination of BV with nivolumab resulted in a 48% complete metabolic response rate for the 46 evaluable patients. This regimen was well tolerated by most patients. One death was possibly related to the combination (cardiac arrest).32
R/R
In a pivotal phase II trial assessing the efficacy of BV in patients with CD30+ R/R HL, overall disease control was achieved in 96% of patients, with CR reported for 34%.33 At 5 years, this trial reported an OS of 41% and a PFS of 22%.34
The efficacy of BV in monotherapy for consolidation therapy after autologous SCT in adult patients with relapsed or primary refractory histologically confirmed HL was evaluated in the AETHERA randomized, double-blind phase III trial. After a median follow-up of 30 months, BV treatment was found to have significantly prolonged PFS to 42.9 months relative to the PFS of only 24.1 for the placebo group.35 PFS at 5 years was 59% in the BV group and 41% in the placebo group.36
The most recent trial comparing the anti-PD1 mAb pembrolizumab in monotherapy versus BV reported even better results, with a statistically significant improvement in PFS to 13.2 months for pembrolizumab, versus 8.3 months for BV but a slightly worse tolerance in the anti-PD1 arm.37 Recent results for BV/anti-PD-1 antibody combinations suggest that such treatments may be the way forward.
Indeed, in phase I/II study, treatment with eight cycles of a combination of BV and nivolumab (3 mg/kg) every 21 days were found to be highly effective in patients with R/R HL, with an objective response rate (ORR) of 85% in the 91 patients studied, and CR achieved in 67%. OS at 3 years was 93%. This combination was also well-tolerated with grade 3 or higher immune-related adverse events (irAE) reported in 7% of patients.38
One phase I/II trial tested nivolumab + BV, ipilimumab (a fully human anti-CTLA-4 mAb) + BV, and a combination of ipilimumab + nivolumab + BV in patients with R/R HL who had relapsed after at least one line of treatment. The ORR were 76%, 89% and 82%, with CR rates of 57%, 61% and 73%, in the ipilimumab + BV, nivolumab + BV and tritherapy groups, respectively. PFS at 1 year was 61% for the ipilimumab + BV group, 70% for the nivolumab + BV group, and 80% for the tritherapy group. Median PFS was 1.2 years for the ipilimumab + BV group but was not reached for the two other arms after a median follow-up of 2.6 years for ipilimumab + BV, 2.4 years for nivolumab + BV and 1.7 years for the tritherapy. The median OS has not yet been reached for any of the groups.39 A phase II trial is currently underway to determine the efficacy and tolerance of these combinations more precisely (NCT01896999).
T-cell lymphomas
Cutaneous T-cell lymphomas are a rare group of NHL with heterogeneous characteristics, characterized by severe pruritus and recurrent infectious complications. The most common forms are mycosis fungoides and Sézary syndrome.40 These lymphomas are generally incurable.
The ALCANZA clinical trial included 131 patients with previously treated cutaneous T-cell lymphoma randomized to BV (1.8 mg/kg once every 3 weeks for up to 16 cycles) or the physician's choice of therapy (oral methotrexate, 5–50 mg once weekly, or oral bexarotene, 300 mg/m2 once daily for up to 48 weeks). The final analysis of the ALCANZA trial revealed a durable, robust clinical benefit of BV, with a significant improvement in objective responses, which lasted at least 4 months (54.7% vs. 12.5%), CR (17.2% vs. 1.6%) and progression-free survival (PFS: 16.7 months vs. 3.5 months) after a median follow-up of 45.9 months.41
Systemic ALCL is a rare, mature T-cell NHL. A phase II trial has been performed to assess the efficacy and safety of BV in patients with R/R systemic ALCL. CD30 positivity and histology were verified by a central pathology review.41 The ORR was 86%, with a CR reported in 57% of patients. The median duration of the response was 12.6 months for OR and 13.2 months for CR. Neutropenia, thrombocytopenia and peripheral sensory neuropathy were the principal grade 3 or 4 adverse events affecting more than 10% of patients.42 After a median observation period of approximately 6 years from the first treatment, no progression was observed beyond 40 months, and median overall survival (OS) was not reached. Moreover, the median PFS was not reached for patients with a CR. Finally, most patients with peripheral sensory neuropathy reported a resolution or improvement at their last assessment.43 It should be noted that one patient with ALCL developed toxic epidermal necrolysis over 90% of the body surface area 7 days after a single administration of BV. Although the mechanism by which BV may have damaged the patient's epidermis is unclear, the imputability of BV could not be excluded.44
The long-term clinical trial data are also encouraging, with a 30% decrease in progression events in peripheral T-cell lymphoma in the BV + CHP (cyclophosphamide, doxorubicin and prednisone) arm relative to the CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) control arm in the ECHELON-2 trial. Median PFS increased from 23.8 months in the CHOP arm to 62.3 months in the BV + CHP arm.45
Others CD30+ malignancies
DLBCL is the most common form of non-Hodgkin lymphoma (NHL).46 In the pre-CAR-T era, a quarter of DLBCL patients relapsing after or refractory to first-line treatment (based on rituximab plus CHOP [R-CHOP]) were eligible for curative salvage chemotherapy followed by autologous stem-cell transplantation (SCT). Treatments for patients not eligible for SCT or for whom salvage regimens failed to remain a matter of serious concern.
For second-line treatment and beyond, BV yielded interesting results in the SGN35-012 study, in patients with relapsing or refractory (R/R) DLBCL. Patients received a median of four cycles of treatment. The overall response rate (ORR) was 44%, including 17% with a complete response (CR) with a median duration of 16.6 months. No statistical correlation could be found between CD30 expression levels and response to BV, or between sCD30 levels and response. However, the median baseline sCD30 concentration was higher in non-responders than in patients with a CR or PR to BV (223 ng/mL vs. 121 ng/mL).47
In combination with lenalidomide, an immunomodulatory agent, the ORR was 57%, with a median response duration of 13.1 months and 35% CR in 37 patients with R/R DLBCL. The BV + lenalidomide regimen was well tolerated, with a toxicity profile consistent with that obtained for their use as single agents. Most patients required granulocyte colony-stimulating factor support because of neutropenia.48 Larger studies with BV have confirmed that neutropenia and peripheral neuropathy are the most frequent adverse effects of this treatment.33, 45
Primary mediastinal B-cell lymphoma (PMBL) is a rare aggressive subtype of DLBCL with poor outcomes in patients with R/R disease. PMBL is characterized by high levels of expression for programmed death ligand 1 (PD-L1) and variable levels of CD30 expression. The CheckMate 436 trial enrolled patients with confirmed R/R PMBL previously treated either by SCT or with two or more chemotherapy regimens if ineligible for SCT. The combination of BV + nivolumab was studied in these patients. After a median follow-up of 11.1 months, the ORR was 73%, with a CR rate of 37%. Treatment-related adverse events were consistent with the safety profiles of the nivolumab and BV treatments used alone. Nivolumab and BV may act in synergy when used together, resulting in a durable high investigator-assessed ORR.49
Human herpesvirus type 8 (HHV8) is associated with three types of cancer: Kaposi sarcoma, Castleman disease and primary effusion lymphoma (PEL). PEL is a rare aggressive B-cell NHL usually associated with human immunodeficiency virus (HIV) and severe immunodeficiency. Classic PEL is characterized by a lymphomatous effusion involving the pleural, pericardial and/or abdominal cavities, with solid tumour masses. This lymphoma has a very poor prognosis. Treatment is based on combined antiretroviral therapy (cART) plus CHOP or CHOP-like chemotherapy.50, 51 PEL does not express pan-B markers, such as CD20, but it generally expresses CD30 and is, therefore, an excellent candidate for anti-CD30 therapies.
In vitro, treatment with BV has been shown to inhibit cell proliferation by inducing G2/M arrest and apoptosis in multiple PEL cell lines.52 Three HIV-infected PEL patients53, 54 and one PEL patient without HIV infection55 were treated with BV after at least one line of chemotherapy. In one HIV-infected patient, a loss of CD30 expression was shown to be associated with a lack of BV efficacy.53 All the other patients responded very well to this treatment, with a sustained CR achieved after 10, 16 and 39 months, respectively.53-55
- Bispecific antibodies (bsAbs)
NK cells are components of the innate immune system. These cytotoxic lymphoid cells play an essential role in immunosurveillance and can mediate effector responses directed against tumour cells. NK cells do not express antigen-specific receptors, but they have activation and effector functions mediated by various receptors, including the CD16a (also called FcγRIII, a low-affinity receptor for the IgG Fc domain involved in antibody-dependent cytotoxicity [ADCC]).57
A bispecific antibody (bsAb) against CD30/CD16 (murine anti-CD30 HRS3 Ab conjugated with the anti-CD16 A9 Ab) was developed, with one arm reportedly binding the CD30 antigen, and the other recruiting NK by binding the CD16 receptor (cf. Figure 2). This bispecific construction aims to lead to the formation of an “immunological synapse”, subsequent activation of NK cells, release of cytotoxic substances, granzyme B and perforin, and finally lysis of CD30-positive cells. The HRS-3/A9 bsAb was shown to activate NK cells and to induce complete remission in SCID mice bearing CD30+ human Hodgkin lymphoma tumours.57
In clinical trials in humans, nine of the 15 patients with refractory HL treated with HRS-3/A9 every 3–4 days (1 mg/m2 to 64 mg/m2/infusion) developed anti-drug antibodies (ADA) that may compromise the efficacy and impact the safety profile. Indeed, four patients suffered allergic reactions on reinfusion and were excluded from further treatment. Complete and partial remission was observed in one patient each.56 The development of this drug was not pursued to move to more innovative bispecific NK-activating antibodies.
Another NK-activating bsAbs under study is AFM13, a first-in-class tetravalent bispecific antibody with two binding sites for CD30 and two for CD16a (anti-CD30 Ab derived from the murine HRS-3 and human anti-CD16a Abs; Figure 2). AFM13 has been shown to enhance NK cell cytotoxicity in vitro and in vivo in mice and cytokine production by IL12/15/18-induced memory-like mature blood NK cells.61
AFM13 was studied in a phase I dose-escalation study (AFM13-101) in 28 patients with heavy prior treatment for R/R CD30+ HL.62 AFM13 was infused weekly for 4 weeks, at doses of 0.01–7 mg/kg. Adverse events were generally mild to moderate. Significant NK cell activation and a decrease in sCD30 levels in peripheral blood were reported, but the best clinical response was a PR, observed in only 11.5% of patients. Following the administration of AFM13 at a dose of ≥1.5 mg/kg, the ORR was 23% and the overall disease control rate was 77% in subjects with heavy prior treatment. ADA was detected in 15 of 28 patients, and they had the potential to neutralize the treatment in 50% of cases. In this study, AFM13 was found to be partially active in patients with disease refractory to BV.60, 62
- Cell and gene therapies
Chimeric antigen receptor-T (CAR-T) cells have been shown to be remarkably active against haematological tumours, particularly for CAR-T cells targeting CD19.
Chimeric antigen receptors (CARs) are engineered so as to target a specific cell-surface molecule. They combine both antigen-binding and T cell-activating functions, while overcoming the constraints of MHC-restricted TCR recognition. CAR molecules are usually created by fusing the single-chain variable fragment (scFv) from a selected antibody to signalling domains.64 Once the CAR-T cell binds to its target, a signalling cascade triggers T cell effector functions, such as the production of perforin and granzyme, which lead to tumour cell death. In the challenging context of R/R HL, CAR-T cell therapy is of considerable interest, and may even prove revolutionary.
The first CAR-T cells targeting CD30 were studied in the late 1990s. They were derived from the monoclonal antibody HRS3 and the Fc-epsilon RI-receptor gamma-chain signalling domain, and they yielded good results in vitro. In pre-clinical data, transfected T cells were activated via the CAR after stimulation with CD30. A specific cytolytic response directed against CD30+ HL cell lines was observed. This response was dependent on the ratio of CAR-T cells to target cells and incubation time. Efficacy was independent of the sCD30 level, as the epitope of CD30 targeted did not seem to be retained in the soluble form.65, 66 Nevertheless, these first-generation CARs were of limited efficacy in vitro, as they did not combine costimulatory signals, such as CD28 or 4-1BB, with a signalling domain.
In a recent phase I/II study, 41 patients with R/R HL were treated with second-generation anti-CD30 CAR-T cells (Figure 2). Three lymphodepletion regimens were used: bendamustine, bendamustine + fludarabine or cytarabine + fludarabine. Anti-CD30 CAR-T cells were injected 2–5 days after the end of lymphodepletion. No overall response was detected in any of the patients with active disease who underwent lymphodepletion with bendamustine alone. Patients with the evaluable disease who received fludarabine-based lymphodepletion had high rates of durable responses, with an ORR of 72%, including a CR in 59% of these patients, and the one-year PFS was 41% (vs. 36% for patients with all types of lymphodepletion considered together).
Ten patients developed grade 1 cytokine release syndrome (CRS) without the need for treatment. No neurotoxicity was observed. The most serious adverse events (grades 3 and 4) were mostly haematological, with grades 3–4 thrombocytopenia persisting for 3 months in four patients.67
In another study testing an anti-CD30 CAR in 18 patients with R/R HL after three different conditioning chemotherapy regimens (fludarabine + cyclophosphamide, gemcitabine + mustargen + cyclophosphamide, or nab-paclitaxel + cyclophosphamide), PR was achieved in 39% of patients and stable disease was achieved in 33%, after observation for at least 2 months after the injection for assessment of the response. In this study, no significant difference was found between the three conditioning regimens. Grade 3 and 4 toxicities occurred in only two of the 18 patients.68
Experiments in vitro and in vivo in mouse models have given promising results for T-cell lymphoma. Anti-CD30 CAR-T cells have been shown to have an efficient cytotoxic effect when cocultured with target cells and to display significant anti-tumour effects after injection into peripheral T-cell lymphoma (PTCL) xenograft tumours.69
CAR-T cell-based combinations have also been considered. One phase II clinical trial combined a single dose of anti-CD30 CAR-T cells with an anti-PD1 Ab administered 14 days later and then every 3 weeks thereafter, in patients with CD30+ lymphomas, after lymphodepletion with fludarabine and cyclophosphamide. In two additional control cohorts, patients received 106/kg and 107/kg CAR-T cells, respectively. The ORR was 100%, with 80% of patients displaying a CR, in the combination cohort, which consisted of five patients (vs. 91.7% and 50%, respectively, for all cohorts considered together). CRS was observed in four of 12 patients, but there was no neurotoxicity. After a median follow-up of 21.5 months, PFS was 45% and OS was 70%. Combination with an anti-PD1 Ab, therefore, appeared to reinforce the action of anti-CD30 CAR-T cells and was very well tolerated.70
A 21-year-old woman with scleronodular HL, initially treated by standard ABVD-based chemotherapy, was treated by anti-CD19 and anti-CD30 CAR-T cells after myeloablaive chemotherapy 10 months later. The patient received three administrations of anti-CD19 CAR-T cells (1.61, 5.20 and 5.20 × 105 cells/kg). The patient's clinical condition improved rapidly. Twelve weeks after, the patient received an injection of anti-CD30 CAR-T cells (1.32 × 106 cells/kg). No severe toxicity was observed after the administration of CAR-T cells. More than 1 year after the end of treatment, no relapse was observed.71
CART-T cell therapy targeting CD30, therefore, appears to be safe, feasible and effective in R/R HL. Interest in this strategy is clearly growing and now extends to other lymphomas. Indeed, several studies are currently enrolling participants with different types of CD30+ lymphomas (see Table 2).
| NCT and phase | Treatment | T of patients included | Type of CD30+ lymphoma | Location |
|---|---|---|---|---|
|
NCT01192464 Phase I |
CAR.CD30 EBV-specific CTLs (autologous) | 18 years and older | All lymphomas | USA (Houston) |
|
NCT04653649 Phase I/II |
HSP-CAR30 (2nd generation CAR-T cells) | 18–70 years |
HL ALCL T-cell lymphoma |
Spain (Barcelona) |
|
NCT04008394 Phase I |
Anti-CD30 CAR-T cells | 18–70 years |
HL ALCL T-cell lymphoma NK-cell lymphoma AITL |
China (Hubei) |
|
NCT04288726 Phase I |
CD30.CAR-EBVST cells (allogeneic) | 18–75 years |
HL NHL ALCL T-cell lymphoma |
USA (Houston) |
|
NCT05320081 Phase 2 |
Anti-CD30 CAR-T cells + camrelizumab | 18–70 years | All lymphomas | China (Hubei, Jiangxi) |
|
NCT04526834 Phase I |
Anti-CD30 CAR-T cells | 18–75 years |
ALCL T-cell lymphoma NK-cell lymphoma DLBCL PMBCL |
USA (Duarte, Nashville, Houston) |
|
NCT02917083 Phase I |
Anti-CD30 CAR-T cells | 12–75 years | All lymphomas | USA (Houston) |
|
NCT05208853 Phase I |
Anti-CD30 CAR-T cells | 18–70 years | All lymphomas | China (Zhejiang) |
|
NCT02690545 Phase I/II |
Anti-CD30 CAR-T cells |
3 years and older | All lymphomas | USA (Chapel Hill) |
|
NCT02259556 Phase I/II |
Anti-CD30 CAR-T cells |
16–80 years | All lymphomas | China (Beijing) |
|
NCT03383965 Phase I |
Anti-CD30 CAR-T cells |
2–80 years | All lymphomas | China (Shandong) |
|
NCT03602157 Phase I |
Anti-CD30 CAR-T cells (with CCR4 co-expression) | 18 years and older | All lymphomas | USA (Chapel Hill) |
|
NCT04083495 Phase II |
Anti-CD30 CAR-T cells |
18–99 years | T-cell lymphoma | USA (Chapel Hill, Winston-Salem) |
- Abbreviations: AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; PMBCL, primary mediastinal large B-cell lymphoma.
CONCLUSIONS
CD30 is an exciting treatment target, given its expression by a large number of lymphomas and the low risk of off-target effects.
The development of anti-CD30 therapy began more than 20 years ago with naked monoclonal antibodies. These antibodies have since been replaced by antibody-drug conjugates, such as brentuximab vedotin, or bispecific antibodies, and, more recently, by cell and gene therapies.
BV was the first anti-CD30 therapy to change clinical practice. This ADC has been extensively studied in R/R haematological malignancies, including R/R HL in particular, and is of significant benefit. T-cell lymphomas also benefit from the impressive durable effects of BV and the ADC is being moved to the frontline in this setting. Added value has also been reported for the addition of BV to first-line treatment with AVD for HL, even in cases of advanced disease. Moreover, the BV + AVD regimen has the advantage of not containing bleomycin, which has been associated with fatal pulmonary toxicity in a few cases.
BV recently gave promising results in combination with anti-immune checkpoint inhibitor antibodies. The combination of an anti-PD1 antibody and BV seems to be synergic in R/R DLBCL/PMBL, and may, therefore, be of considerable interest for the treatment of R/R HL, as suggested by recent phase I/II studies. The results appeared to be significantly improved by the addition of a second anti-immune checkpoint inhibitor, but more extended use would require a tolerance profile definition. More results should soon become available to consolidate the published findings and may lead to changes in clinical practice for R/R HL.
Resistance has been reported in cases of repeated BV exposure, in the form of an increase in drug transporter protein expression and MMAE resistance. Moreover, BV may have off-target effects, as shown by the dose-limiting and cumulative peripheral neuropathy observed in some cases after treatment, probably due to the anti-tubulin action of MMAE. There have also been reports of pancreatitis and progressive multifocal leukoencephalopathy.
Any anti-CD30 therapy is, theoretically, highly relevant for CD30+ cancers, but the construction of the treatment is of particular importance. Indeed, only moderately good clinical results have been obtained with anti-CD30 bsABs recruiting NK cells, possibly due to immunization of the patients with the parts of the antibody of murine origin. Further studies are also required to determine the relationship between clinical activity and biological indicators, such as NK cell counts.
For CAR-T cells, the use of scFV-based construction may help to limit the ADA phenomenon to some extent. Moreover, this design also ensures independence from the HLA system and long-term persistence, raising hopes that it may be possible to achieve a PFS plateau at some point. The considerable efficacy of this cell-and-genetic therapy has been clearly demonstrated for CD19+ cancers, paving the way for exciting new treatment opportunities. CAR-T cells are now the standard of care for some R/R non-Hodgkin lymphomas (DLBCL, mediastinal lymphoma, mantle cell lymphoma, follicular lymphoma). The “one-shot” nature of this treatment is a clear advantage. However, toxicity – such as cytokine release syndrome and neurotoxicity in particular – remains an obstacle to the widespread use of this treatment in clinical practice.
In addition, the low amount and quality of patient T cells as well as the time and cost of CAR T cell synthesis could be obstacles for autologous CAR T cells. Other approaches are therefore currently investigating, such as the production of universal CAR T cells by TCR and HLA class I genome editing using CRISPR/Cas9 system. This technique may open the way to numerous perspectives. For example, universal CAR T cells can also be made resistant to immune check-points.72
Anti-CD30 CAR-T cells have given encouraging results, and combinations with other types of immunotherapies are already emerging. The efficacy of anti-CD30 CAR-T cells appears to be enhanced by combination with anti-PD1 immunotherapy while remaining very well tolerated. However, very few patients have been treated to date and further studies are therefore required to confirm these findings. A potential synergistic effect of these cells in combination with two immune checkpoint inhibitors (for example with a combination of anti-PD1 and anti-CTLA-4 Abs) should be studied. No neurotoxicity has been reported to date, possibly due to the absence of CD30 expression in the CNS.
The selection of CD30-negative cell clones has rarely been reported but could potentially limit the use of anti-CD30 therapeutics. A loss of CD30 expression was reported in a patient with R/R HL after treatment with BV followed by anti-CD30 CAR-T cells.73 A downregulation of CD30 after BV therapy has also been noted in R/R ALCL.74-76 For CAR-T cells, the nature of the costimulation domain (CD28 or 4–1 BB) may be of importance, as already reported for CD19 CAR-T cell-based regimens.77 In addition, the impact on outcomes of CD30 expression and CD30 loss should be properly refined during the trials.
In conclusion, anti-CD30 therapy is booming. Considerable benefits have already been reported for some very aggressive cancers with a poor prognosis, together with significant improvements in more common R/R diseases. Further studies are required to determine the best strategy, particularly for rarer CD30+ cancer, such as ALCL or PEL, for which there is currently no standard of care. Results are awaited with interest for new constructions such as NK combined with AFM13 or dual and universal CAR T cells.
In the near future, anti-CD30 strategies will probably involve a combination with immunotherapies, including immune checkpoint inhibitors in particular.
AUTHOR CONTRIBUTIONS
MV and ET searched and analysed the data, wrote the paper and realized the figures. JPS, FLB and AGM proofread and validated the paper. The authors thank Sophie Sayon and Pr. Vincent Calvez for their help in the realization of this paper.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
All authors have no direct conflict of interest with the paper.
Open Research
DATA AVAILABILITY STATEMENT
References for this review were identified through searches of PubMed with the search terms “CD30”, “CAR”, and “brentuximab vedotin” for articles published from 1980 until June 2022. Articles were also identified through searches of the authors' own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.




