Outcome of early treatment of SARS-CoV-2 infection in patients with haematological disorders
Summary
Outcome of early treatment of COVID-19 with antivirals or anti-spike monoclonal antibodies (MABs) in patients with haematological malignancies (HM) is unknown. A retrospective study of HM patients treated for mild/moderate COVID-19 between March 2021 and July 2022 was performed. The main composite end-point was treatment failure (severe COVID-19 or COVID-19-related death). We included 328 consecutive patients who received MABs (n = 120, 37%; sotrovimab, n = 73) or antivirals (n = 208, 63%; nirmatrelvir/ritonavir, n = 116) over a median of two days after symptoms started; 111 (33.8%) had non-Hodgkin lymphoma (NHL); 89 (27%) were transplant/CAR-T (chimaeric antigen receptor T-cell therapy) recipients. Most infections (n = 309, 94%) occurred during the Omicron period. Failure developed in 31 patients (9.5%). Its independent predictors were older age, fewer vaccine doses, and treatment with MABs. Rate of failure was lower in the Omicron versus the pre-Omicron period (7.8% versus 36.8%, p < 0.001). During the Omicron period, predictors of failure were age, fewer vaccine doses and diagnosis of acute myeloid leukaemia/myelodysplastic syndrome (AML/MDS). Independent predictors of longer viral shedding were age, comorbidities, hospital admission at diagnosis, NHL/CLL, treatment with MABs. COVID-19-associated mortality was 3.4% (n = 11). The mortality in those who developed severe COVID-19 after early treatment was 26% in the Omicron period. Patients with HM had a significant risk of failure of early treatment, even during the Omicron period, with high mortality rate.
Abbreviations
-
- AML/MDS
-
- acute myeloid leukemia or mylodysplastic syndrome
-
- B+E
-
- bamlanivimab and etesevimab
-
- CAR-T
-
- chimaeric antigen receptor T cell therapies
-
- C+I
-
- casirivimab and imdevimab
-
- CLL
-
- chronic lymphocytic leukemia
-
- CML
-
- chromic myeloid leukemia
-
- HM
-
- hematological malignancies
-
- HR
-
- hazard ratio a
-
- HSCT
-
- haematopoietic stem cell transplant
-
- IQR
-
- interquartile range
-
- ISS
-
- the Italian Institute of Healthcare
-
- MABS
-
- anti-spike monoclonal antibodies
-
- MDS
-
- myelodysplastic syndrome
-
- NHL
-
- non-Hodgkin lymphoma
-
- NHL/CLL
-
- non-Hodgkin lymphoma or chronic lymphocytic leukemia
-
- sHR
-
- subdistribution hazard ratio
INTRODUCTION
Patients with haematologic malignancies (HM), including haematopoietic stem cell transplant (HSCT) and chimaeric antigen receptor T-cell therapy (CAR-T) recipients infected with SARS-CoV-2 have higher risk of progressing to severe COVID-19 compared to the general population.1 Although the prognosis of COVID-19 has significantly improved over time due to better management, vaccination, available treatment options and changes in viral virulence, patients with HM remain among those with worse prognosis and an early case fatality rate of approximately 14%.2-5 Indeed, during Omicron predominance, 52% (309/593) of HM patients with COVID-19 were admitted to hospital, with an overall mortality of 16.5% among hospitalized patients and 8.6% in the whole cohort.6 This is still much higher than in the general population with Omicron infection (0.11% at day 28).7 In addition, the negative impact of COVID-19 in the immunocompromised should be considered on a timeframe longer than 28 days, since delayed mortality might be present.8
New treatment options have been developed and introduced in Italy starting from March 2021 for early treatment of mild to moderate COVID-19 in patients at risk for progression to severe disease: anti-spike monoclonal antibodies (MABs),9-11 and antivirals such as molnupiravir, nirmatrelvir/ritonavir and remdesivir.12-14 Although the immunocompromised might be the population that is expected to gain maximum benefit from early treatment, data are limited since these patients have been excluded or underrepresented in the randomized trials.15, 16
Negative impact of SARS-CoV-2 infection in the HM population is not limited to a high rate of progression to severe COVID-19 and high mortality, but comprises also prolonged viral shedding, with possible negative consequences for withdrawing or postponing chemotherapy.17 In 77 studies in the general population the median duration of SARS-CoV-2 RNA positivity was 18 days after the onset of symptoms.18, 19 There are numerous reports of persistent RNA positivity and persistent or recurring symptomatic SARS-CoV-2 infection in HM patients, mainly in those treated with B-cell-depleting therapies.17, 20-23 In 382 HM patients infected between March 2020 and March 2021, the rate of SARS-CoV-2 RNA detection beyond day 30 was 13.9%.22 Little is known about the rate and predictors of prolonged SARS-CoV-2 shedding in HM patients receiving early therapy.
The aim of our study is to report the outcomes of early treatment of mild/moderate COVID-19 in patients with HM, and to identify the predictors of poor outcomes.
METHODS
Study design
A retrospective study included all consecutive patients with HM who received early therapy for mild/moderate (i.e. not requiring oxygen therapy) COVID-19 from March 2021 to July 2022 in two university hospitals in Italy. Only the first treated COVID-19 episode was included. Patients who received early treatment with both antivirals and MABs were excluded.
Patients were mainly tested for the presence of new onset of SARS-CoV-2 symptoms, either respiratory or systemic. Patients who resulted positive during screening after close exposure or prior to chemotherapy or HSCT were evaluated for the presence of any signs or symptoms compatible with SARS-CoV-2 infection. Treatment was provided to all patients with SARS-CoV-2 symptoms and to all patients who were already admitted to hospital for chemotherapy or HSCT in whom we could not exclude that already present aspecific symptoms were caused or aggravated by SARS-CoV-2 infection.
The testing was performed either at the hospital by polymerase chain reaction (PCR) (e.g., in outpatient service or during admission to the ward) or in the community (usually with antigen tests) either at home, or, more frequently, in a pharmacy, which in Italy were recognized as official settings for COVID-19 diagnosis and notification.
In both centres, all patients received counselling regarding the appropriate preventive measures, early symptom recognition, the importance of early testing, and the need to report promptly to the caring haematologist any positive results, so appropriate treatment could be prescribed by an infectious-diseases consultant.
The study was approved by hospital the Ethics Committee (283/2020). The need for signed informed consent was waived due to the retrospective nature of the study.
Definitions
SARS-CoV-2 infection was defined as new detection of SARS-CoV-2 RNA or antigen in respiratory samples. Mild/moderate disease was defined as grade less than 4 on the WHO COVID Outcomes Scale, that is, not requiring oxygen therapy for COVID-19.
The duration of SARS-CoV-2 shedding was defined as time from the first positivity to the first negative result obtained with either PCR or antigen assay. In both centres, follow-up testing was performed usually every 5–7 days by antigen assay in the outpatient setting and by PCR in inpatients, until the first negative result, due to the national policy for immunocompromised patients which required a negative result for ending isolation. Routine testing after the first negative swab was not performed in all patients. New SARS-CoV-2 infection was defined as PCR or antigen positivity more than 90 days after the onset of clinically and virologically resolved infection.24
Early treatment was defined as treatment with nationally authorized MABs or antivirals administered as soon as possible after SARS-CoV-2 diagnosis and before developing respiratory failure which required oxygen treatment due to COVID-19 infection. The nationally authorized time limits for early infection varied: 10 days from symptom onset for MABs such as bamlanivimab + etesevimab, casirivimab + imdevimab or sotrovimab, seven days for remdesivir and five days for nirmatrelvir/ritonavir and molnupiravir, according to each drug's registration trial.
The oral antivirals nirmatrelvir/ritonavir and molnupiravir were authorized in Italy on 26 November 2021, remdesivir for early treatment on 30 December 2021. In both centres, all the nationally authorized early-treatment drugs were available without shortages throughout the whole study period, and the choice of treatment relied on clinical decision based on patient's characteristics (i.e., severe renal failure, drug–drug interactions, hospital admission), with nirmatrelvir/ritonavir being the first choice followed by remdesivir and then by molnupiravir, based on data on efficacy, pharmacological characteristic and current guidelines.16, 25 In case of MABs, activity against the currently circulating variant was considered. During the months when both nirmatrelvir/ritonavir and MABs were available, nirmatrelvir/ritonavir was preferred if not contra-indicated and if oral administration was feasible. Patients could be treated either in the hospital or as outpatients. Both centres had the possibility of administering three days of remdesivir as outpatient intravenous infusion.
COVID-19 death was defined as death while SARS-CoV-2-positive and no other known imminent life-threatening condition. Progression to oxygen therapy on the same day as early treatment was included in the analysis as failure on day +1. Late failure was defined as occurring more than 10 days after starting the early treatment.
The distribution of predominant variants was established based on national data from the Italian Institute of Healthcare (ISS).26, 27
End-points and data collected
The main outcome was a composite outcome of treatment failure defined as progression to severe COVID-19 requiring oxygen supplementation, corresponding to grade 4 or higher on the WHO COVID Outcomes Scale, or COVID-19-related death. Secondary outcomes were the length of SARS-CoV-2 positivity, COVID-19-associated mortality and overall 90-day mortality.
The following data were collected: age; gender; underlying disease and its stage (remission: complete, partial or non-malignant disorder, versus active malignancy); previous chemotherapy, particularly if anti-CD20 agents were included; previous HSCT/CAR-T treatment; comorbidities such as diabetes mellitus, chronic renal or hepatic failure, cardiopathy, obesity, HIV infection; type of early treatment and the day of its start; previous vaccination and number of doses; clinical and virological outcomes, virological parameters and treatments.
Statistical analysis
Categorical variables were presented as absolute numbers and their relative frequencies, continuous variables as median and min–max values, and interquartile range (IQR) for selected variables and compared, respectively, with the chi-squared test (or Fisher's exact test when appropriate) and the Mann–Whitney test.
The rate of failure, COVID-19-related mortality and overall mortality were assessed in a Cox proportional hazard model. Variables associated with the outcome at univariate analyses with p < 0.2 were introduced into a Cox regression multivariable model with backwards conditional stepwise entry method. The independent impact of selected variables on outcome was presented as an adjusted survival curve from the Cox regression analysis.
The time to outcome was calculated from the day of early treatment to the day of development of oxygen-requiring COVID-19 or COVID-19-related death, whichever occurred first.
Predictors of the time to first negative SARS-CoV-2 swab were assessed in Cox regression analyses, calculating cause-specific unadjusted and adjusted hazard ratio (HR) values. The cumulative risk of becoming SARS-CoV-2-negative over time was presented as a cumulative incidence curve with death while SARS-CoV-2-positive considered as a competing event using a multivariable Fine–Grey model.
A p-value of 0.05 or less was considered significant. Descriptive and Cox regression analyses were performed using the Statistical Package for Social Sciences (SPSS for Windows ver.21.0; SPSS Inc.). Competing risk analysis was performed with R (R Statistical Software program, version 3.6.0; R Foundation for Statistical Computing).
RESULTS
Patients
Overall, 331 patients received early treatment during the study period. Since three patients received both antiviral and MABs, 328 patients were finally included. Non-Hodgkin lymphoma (NHL) was the most frequent diagnosis (33.8%), 27% had undergone HSCT or CAR-T, and 65% had received three or four doses of COVID-19 vaccine (Table 1).
| Total | Patients treated with MABs | Patients treated with an antiviral | p | |
|---|---|---|---|---|
| N = 328 | N = 120 (36%) | N = 208 (64%) | ||
| Age, years, median (min–max) | 66 (16–89) | 62 (16–85) | 68 (22–89) | 0.007 |
| Male sex n (%) | 195 (59.5) | 72 (60) | 123 (59.1) | 0.87 |
| Diabetes mellitus | 33 (10.1) | 9 (7.5) | 24 (11.5) | 0.242 |
| Chronic renal failure | 34 (10.4) | 12 (109) | 22 (10.6) | 0.869 |
| Number of comorbidities | 1 (0–5) | 1 (0–5) | 1 (0–5) | 0.019 |
| Underlying disease | ||||
| Acute myeloid leukaemia | 51 (15.5) | 24 (20) | 27 (13) | |
| Acute lymphoid leukaemia | 13 (4) | 7 (5.8) | 6 (2.9) | |
| Non-Hodgkin lymphoma | 111 (33.8) | 40 (33.3) | 71 (34.1) | |
| Hodgkin disease | 14 (4.3) | 5 (4.2) | 9 (4.3) | |
| Chronic lymphocytic leukaemia | 33 (10.1) | 7 (5.8) | 26 (12.5) | |
| Multiple myeloma | 78 (23.8) | 28 (23.3) | 50 (24) | |
| Myelodysplastic syndrome | 9 (2.7) | 3 (2.5) | 7 (2.9) | |
| Myelofibrosis | 9 (2.7) | 5 (4.2) | 4 (1.9) | |
| Other (aplastic anaemia, 4; CML, 3; other 3) | 10 (3) | 1 (0.8) | 9 (4.3) | |
| Underlying disease, acute leukaemia versus other | 64 (19.2) | 31 (25.8) | 33 (15.9) | 0.028 |
| Underlying disease in remission | 144 (43.9) | 53 (44.2) | 91 (43.8) | 0.942 |
| Previous HSCT/CAR-T | 89 (27.1) | 39 (32.5) | 50 (24) | 0.097 |
| Allogeneic | 49 (14.9) | 22 (18.3) | 27 (13) | |
| Autologous | 37 (11.3) | 16 (13.3) | 21 (10.1) | |
| CAR-T therapy | 3 (1) | 1 (1) | 2 (1) | |
| GvHD, if applicable | 18/49 (36.7) | 10/22 (45.5) | 8/27 (29.6) | |
| Time from HSCT/CAR-T, months | 13 (0–225.5) | 10.2 (1–201) | 18.4 (0–225.5) | 0.244 |
| Recent (within 12 months) HSCT/CAR-T | 42 (12.8) | 21 (17.5) | 21 (10.1) | 0.053 |
| Recent (within 12 months) chemotherapy | 218 (66.5) | 86 (71.7) | 132 (63.5) | 0.13 |
| Recent (within 12 months) anti-CD20 treatment | 66 (20.2) | 26 (21.8) | 40 (19.2) | 0.57 |
| Anti-SARS-CoV-2 vaccination | 330 (91.7) | 98 (81.7) | 202 (97.6) | <0.001 |
| Median number of doses | 3 (0–4) | 2 (0–4) | 3 (0–4) | 0.014 |
| Hospital admission at the time of SARS-CoV-2 diagnosis |
42 (12.8) |
30 (25) |
12 (5.8) |
<0.001 |
| For chemotherapy | 27 (8.2) | 18 (15) | 9 (4.3) | |
| For other reasons | 26 (4.6) | 12 (10) | 3 (1.4) | |
| Time from symptoms to treatment, days, median (min–max) | 2 (0–13) | 2 (0–10) | 2 (0–13) | 0.769 |
| Time from SARS-CoV-2 diagnosis to treatment, median (min–max) | 1 (0–9) | 1 (0–9) | 1 (0–7) | 0.161 |
| Treatment with antiviral | - | - | ||
| Nirmatrelvir/r | 116 (55.8) | 116 (55.8) | ||
| Remdesivir | 59 (28.4) | 59 (28.4) | ||
| Molnupiravir | 33 (15.9) | 33 (15.9) | ||
| MABs | - | - | ||
| B + E | 24 (20) | 24 (20) | ||
| C + I | 23 (19.2) | 23 (19.2) | ||
| Sotrovimab | 73 (68.8) | 73 (68.8) | ||
| Period of treatment | <0.0001 | |||
| Before Omicron | 19 (5.8) | 19 (15.8) | 0 | |
| Omicron predominance | 309 (94.2) | 101 (84.2) | 208 (100) |
- Abbreviations: B + E, bamlanivimab and etesevimab; C + I, casirivimab and imdevimab; CAR-T, chimaeric antigen receptor T-cell therapies; CML, chronic myeloid leukaemia, HSCT, haematopoietic stem cell transplant; MABs, monoclonal antibodies against spike protein of SARS-CoV-2; nirmatrelvir/r, ritonavir-boosted nirmatrelvir.
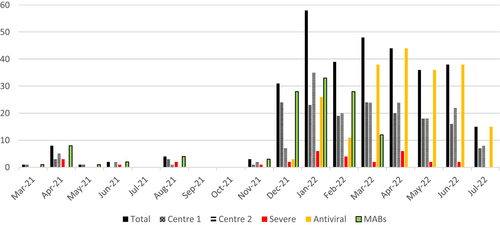
Most infections occurred after December 2021, confirming higher transmissibility and partial immune escape of the new Omicron variants (Figure 1).
Early treatment and its outcome
Overall, 208 (63%) patients received an antiviral and 120 (37%) MABs, on median two days after symptom onset (min–max, 0–13; IQR, 2–4). In particular, time to treatment was more than seven days for four (1.2%) patients, with one receiving nirmatrelvir/ritonavir on day 13 because of logistical difficulties.
The characteristics of patients receiving different treatments is shown in Table 1. Over time there was a change in the choice of early treatment provided (p < 0.0001) (Figure 1). No severe side effects of early treatment were observed, and none of patients discontinued it due to intolerance.
Treatment failure occurred in 31 patients (9.5%); 19 were treated with MABs and 12 with antivirals, with respective rates of failure of 15.8% and 5.8%. Thirty patients required oxygen therapy, and among them 10 (33%) required non-invasive and five (17%) invasive ventilation. One patient experienced sudden death without hospital admission on day 7. Among 31 patients experiencing failure, 15 (48%) developed late failure.
Overall, time to failure was eight days after treatment start (min–max, 1–42). Median time to failure after treatment was 14.5 days (min–max 2–42) for antivirals and three days for MABs (min–max 1–32, p = 0.038). Eight patients, all treated with MABs, developed failure within 24 h of treatment (which rapidly improved in one case). They received early treatment later than those who experienced the failure more than 24 h after starting treatment (median time from symptom onset four versus two days, p = 0.026), but still all within seven days from symptom onset.
Predictors of treatment failure
The univariate analysis of predictors of failure is shown in Table 2. Multivariable analysis confirmed higher risk of failure in case of older age, lower number of vaccine doses and treatment with MABs (Table 3). Figure 2 shows risk of failure according to treatment type in an adjusted survival curve.
| Rate of failure, n = 31 (9.5%) | Unadjusted cause specific HR | 95% CI | p | |
|---|---|---|---|---|
| Age, years, median (min–max) | 71 (22–85) | 1.035 | 1.004–1.067 | 0.027 |
| Male sex n (%) | 18 (9.2) | 0.934 | 0.458–1.907 | 0.852 |
| Diabetes mellitus | 5 (15.2) | 1.807 | 0.694–4.707 | 0.226 |
| Chronic renal failure | 5 (14.7) | 1.745 | 0.67–4.545 | 0.254 |
| Number of comorbidities | 1 (0–5) | 1.232 | 0.922–1.644 | 0.158 |
| Underlying diseasea | 0.056 | |||
| AML/MDS | 10 (16.7) | 1.00 | ||
| NHL/CLL | 15 (10.4) | 0.624 | 0.28–1.389 | |
| Other | 6 (4.8) | 0.289 | 0.105–0.795 | |
| Active diseases versus in remission | 21 (11.4) | 1.711 | 0.805–3.633 | 0.162 |
| Hospital admission at SARS-COV-2 diagnosis | 9 (21.4) | 2.962 | 1.364–6.433 | 0.006 |
| Previous HSCT/CAR-T | 9 (10.1) | 1.089 | 0.501–2.365 | 0.829 |
| Allogeneic | 6 (12.2) | |||
| Autologous | 2 (5.4) | |||
| CAR-T | 1 (33.3) | |||
| Recent (within 1 year) HSCT/CAR-T | 6 (14.3) | 1.618 | 0.664–3.943 | 0.209 |
| Recent (within 1 year) chemotherapy | 19 (8.8) | 0.769 | 0.373–1.587 | 0.477 |
| Recent (within 12 months) anti-CD20 treatment | 4 (6.1) | 0.556 | 0.194–1.589 | 0.273 |
| Days from symptoms to treatment, median | 3 (0–7) | 1.053 | 0.866–1.279 | 0.606 |
| Treatment | 0.004 | |||
| MABs | 19 (15.8) | 1.00 | ||
| B + E | 4 (16.7) | |||
| C + I | 6 (26.1) | |||
| Sotrovimab | 9 (12.3) | |||
| Antivirals | 12 (5.8) | 0.348 | 0.169–0.717 | |
| Nirmatrelvir/r | 6 (5.2) | |||
| Remdesivir | 2 (3.4) | |||
| Molnupiravir | 4 (12.1) | |||
| Anti-SARS-CoV-2 vaccination, n (%) | 0.099 | |||
| No | 5 (18.5) | 1.00 | ||
| Yes | 26 (8.7) | 0.447 | 0.172–1.165 | |
| Number of doses, median | 2 (0–4) | 0.661 | 0.492–0.888 | 0.006 |
| Number of doses | 0.001 | |||
| 0–1 | 10 (23.8) | 1.00 | ||
| 2–4 | 21 (7.3) | 0.282 | 0.133–0.6 | |
| Period of treatment | <0.0001b | |||
| Before Omicron | 7/19 (36.8%) | 1.00 | ||
| Omicron | 24/309 (7.8%) | 0.18 | 0.078–419 |
- The variables which were included in the multivariate analyses are indicated in bold.
- Abbreviations: AML, acute myeloid leukaemia; B + E, bamlanivimab and etesevimab; C + I, casirivimab and imdevimab; CAR-T, chimaeric antigen receptor T-cell therapies; CLL, chronic lymphocytic leukaemia; HM, haematological malignancy; HR, hazard ratio; HSCT, haematopoietic stem cell transplant; MABs, anti-spike protein monoclonal antibodies; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma; nirmatrelvir/r, ritonavir-boosted nirmatrelvir. Continuous variables are expressed as median and min–max.
- a Rate of failure for specific HM: MDS 22% (n = 2); AML 15.7% (n = 8); CLL 15.2% (n = 5); NHL 9% (n = 10); acute lymphoblastic leukaemia 7.7% (n = 1); multiple myeloma 6.4% (n = 5).
- b Variable not included in the multivariable model since separate analysis was performed for the Omicron period.
| Model covariate | Adjusted cause specific HR | 95% CI | p | Adjusted cause specific HR | 95% CI | p |
|---|---|---|---|---|---|---|
| All patients (n = 328) | Patients during the Omicron period (n = 309) | |||||
| Age | 1.055 | 1.02–1.09 | 0.002 | 1.052 | 1.014–1.092 | 0.007 |
| Underlying disease | 0.063 | 0.037 | ||||
| AML/MDS | 1.00 | 1.00 | ||||
| NHL/CLL | 0.772 | 0.336–1.776 | 0.699 | 0.281–1.74 | ||
| Other | 0.303 | 0.11–0.839 | 0.177 | 0.047–0.668 | ||
| Treatment with antivirals versus MABs | 0.432 | 0.193–0.968 | 0.042 | 0.549 | 0.23–1.31 | 0.177 |
| Number of doses of vaccine | 0.628 | 0.428–0.921 | 0.017 | 0.561 | 0.359–0.877 | 0.011 |
- Abbreviations: AML, acute myeloid leukaemia; CI, confidence interval; CLL, chronic lymphocytic leukaemia; HR, hazard ratio; MABs, anti-spike protein monoclonal antibodies; MDS, myelodysplastic syndrome; NHL, non-Hodgkin lymphoma.
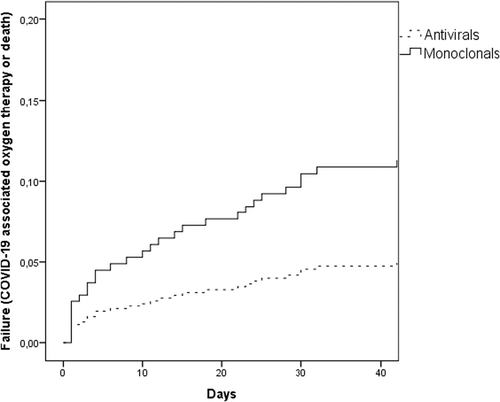
The rate of failure was significantly higher in pre-Omicron versus Omicron period (7/19, 36.8% vs 24/309, 7.8%; p < 0.001). When the analyses were restricted to the Omicron period, the rate of failure was 5.8% (12/208) for antivirals and 11.8% (12/101) for MABs (p = 0.06). In multivariate analysis the independent predictors of failure in the Omicron period were older age, number of vaccine doses, and diagnosis of acute myeloid leukaemia/myelodysplastic syndrome (AML/MDS) (Table 3).
Considering only 97 patients infected during Omicron BA.1 predominance, when all but one MABs-treated patients received sotrovimab which is active against BA.1, the difference in failure rate was no longer significant: 13.1% (8/61) for MABs vs 5.6% (2/36) for antivirals, p = 0.177.
Length of viral shedding
Data on follow-up swabs were available for 318 (97%) patients. Among them, 12 never resolved SARS-CoV-2 infection, and all died on median 29 days after the diagnosis (min–max, 6–159). In 301 patients who resolved SARS-CoV-2 infection, the median time to the first negative swab was 14 days from SARS-CoV-2 infection diagnosis (min–max, 1–112): 81 were still positive by day 21 (17%), and 37 by day 30 (12%).
Patients treated with antivirals, compared to MABs, had significantly shorter viral shedding: median 12 (min–max, 1–104) vs 20 days (min–max, 3–159).
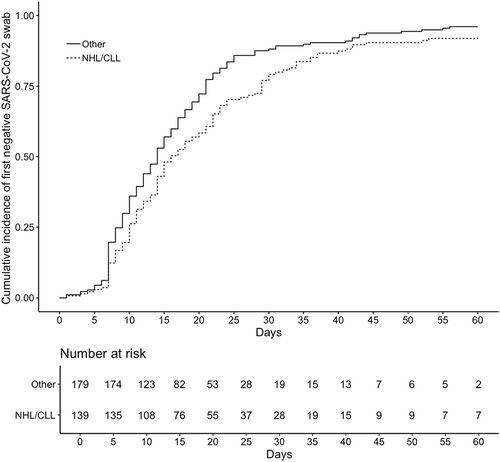
Independent predictors of longer time to first negative swab were: male sex, comorbidities, having NHL or chronic lymphocytic leukaemia (CLL), hospital admission at the time of SARS-CoV-2 diagnosis and early treatment with MABs (Table 4). The same independent predictors were identified also when only the Omicron period was considered (data not shown).
| Unadjusted cause specific HR | 95% CI | p | Adjusted cause specific HR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Age, years | 0.997 | 0.99–1.004 | 0.420 | |||
| Male sex | 0.789 | 0.627–0.994 | 0.044 | 0.780 | 0.619–0.985 | 0.037 |
| Underlying diseasea | 0.021 | 0.001 | ||||
| NHL/CLL | 0.763 | 0.606–0.961 | 0.664 | 0.524–0.841 | ||
| Other | 1.00 | |||||
| Active diseases versus in remission | 0.989 | 0.786–1.243 | 0.922 | |||
| No. of comorbidities | 0.931 | 0.839–1.033 | 0.178 | 0.894 | 0.803–0.996 | 0.042 |
| Hospital admission at the time of SARS-COV-2 diagnosis | 0.618 | 0.439–0.870 | 0.006 | 0.660 | 0.461–0.944 | 0.023 |
| Recent (within 1 year) HSCT/CAR-T | 0.809 | 0.572–1.145 | 0.232 | |||
| Recent (within 1 year) chemotherapy | 1.062 | 0.834–1.353 | 0.626 | |||
| Anti-CD20 within 6 months | 0.822 | 0.618–1.094 | 0.178 | - | ||
| Days from symptoms to treatment | 1.006 | 0.946–1.007 | 0.852 | |||
| Treatment | <0.0001 | <0.001 | ||||
| Antivirals | 1.00 | |||||
| MABs | 0.599 | 0.472–0.761 | 0.575 | 0.445–0.742 | ||
| Number of doses of anti-SARS-CoV-2 vaccine | 1.142 | 1.014–1.286 | 0.028 | - | ||
| Period of treatment | 0.13 | - | ||||
| Before Omicron | 1.00 | |||||
| Omicron | 1.514 | 0.885–2.592 |
- Note: Analysis performed for 318 patients for whom the information on follow-up testing was available. In bold values that are statistically significant. Higher HR refers to probability of shorter time to the first negative result, hence HR < 1 identifies predictors of longer shedding.
- Abbreviations: CAR-T, chimaeric antigen receptor T-cell therapies; CI, confidence interval; CLL, chronic lymphocytic leukaemia; HR, hazard ratio; HSCT, haematopoietic stem cell transplant; MABs, anti-spike protein monoclonal antibodies; NHL, non-Hodgkin lymphoma.
- a With AML/MDS patients as reference group, NHL/CLL patients had longer time to first negative and patients with other diseases had shorter time to first negative, thus, NHL/CLL were compared to all other patients.
The effect of the type of underlying disease on the time to negativity is shown in an adjusted cumulative incidence curve derived by a competing-risk analysis using a multivariable Fine̶–Grey model (Figure 3).
Mortality
Covid-19 related mortality
Overall, COVID-19-associated mortality was 3.4% (n = 11/328; 4/19, 21% in pre-Omicron and 7/309, 2.3% during Omicron period). Among these 11 patients, 10 had experienced treatment failure with hospitalization and oxygen requirement and death occurred after a median of 42.5 days (min–max, 6–143) from SARS-CoV-2 diagnosis, and 33 days (min–max, 5–108) after the need for oxygen therapy. One patient died on day 7 after diagnosis without hospital admission and without respiratory failure. Patients who died due to COVID-19 had a median age of 71.5 (min–max, 58–84), 5 had AML/MDS (one in remission after allogeneic HSCT, four with active disease, among them one after allogeneic HSCT), five had non-Hodgkin lymphoma or chronic lymphocytic leukaemia (NHL/CLL, four in remission, three under treatment with ibrutinib) and one multiple myeloma with disease relapsed after autologous HSCT. The multivariate analysis of predictors of COVID-19 related mortality is shown in Table 5.
| Adjusted cause specific HR | 95% CI | p | |
|---|---|---|---|
| COVID-19 associated mortality | |||
| Age, years | 1.068 | 1.011–1.129 | 0.012 |
| AML/MDS versus other diseases | 3.564 | 1.055–12.039 | 0.041 |
| Early treatment with antivirals versus MABs | 0.434 | 0.124–1.518 | 0.191 |
| Omicron period versus pre-Omicron | 0.121 | 0.034–0.437 | 0.001 |
| Overall 90-day mortality | |||
| Age, years | 1.056 | 1.015–1.099 | 0.007 |
| AML/MDS versus other diseases | 5.172 | 1.991–13.437 | 0.001 |
| Omicron period versus pre-Omicron | 0.237 | 0.076–0.742 | 0.013 |
- Abbreviations: AML/MDS, acute myeloid leukaemia and myelodysplastic syndrome; CI, confidence interval; HR, hazard ratio; MABs, anti-spike protein monoclonal antibodies.
When considering only the group of patients who experienced treatment failure with oxygen requirement (n = 30), COVID-19-associated mortality rate was 33% (n = 10, five with early and five with late failure) for the whole period, and 57% (4/7) for the pre-Omicron and 26% (6/23) for the Omicron period.
Overall mortality
Overall, nine (2.7%), 18 (5.5%) and 28 patients (8.5%) died at 30, 90 and 180 days after SARS-CoV-2 infection respectively. Independent predictors of 90-day overall mortality are shown in Table 5.
DISCUSSION
This study analysed the efficacy of early treatments for COVID-19 in a large cohort of HM patients over a 16-month period during which different viral variants emerged and became predominant. Looking at the distribution of included patients, we noted an important increase in the number of patients receiving early treatment for COVID-19 during Omicron predominance, despite unchanged policies of early testing and early treatment since March 2021, which demonstrates higher transmissibility and capacity to evade prior vaccine-induced humoral immunity.28 These data are in line with national data on rates of new SARS-CoV-2 cases in the same period, which showed a marked increase in infections from December 2021 onwards, when the Omicron variant became dominant in Italy.29 In accordance with the international and national recommendations, the use of available MABs was progressively discontinued due to the reduction in their activity against the emerging variants.30
Clinical features of patients included were comparable to the data available in the literature.1, 4 The rate of progression to severe COVID-19 requiring oxygen was significantly lower than during the pretreatment periods, but still reached 9.5% in the whole population, and 21% in the pre-Omicron period. Indeed, even though the rate of early treatment failure was lower during the Omicron period, it was still 7.8%, which is much higher than in the general population in the same period (1.2%–1.4%), and even higher than in the treatment arm of pivotal studies performed before the Omicron period in the general at-risk population (7.3% for molnupiravir, 0.77% for nirmatrelvir/ritonavir, 0.7% for remdesivir and 1% for sotrovimab).11-14 Our rate was only slightly lower to what was previously reported in more heterogeneous cohorts of patients with HM treated with MABs.31, 32
The probability of failure of early treatment was higher in case of older patients, lower number of previous doses of COVID-19 vaccine and treatment with MABs compared to antivirals. The reasons for lower efficacy of MABs compared to antivirals might be related to antiviral potency; however, during the study period the following changes took place and might have contributed to the lower rate of failure in the more recent period: (1) more patients vaccinated with booster doses; (2) availability of antivirals for early treatment much later than the availability of MABs; and (3) emerging variants with lower virulence but also lower susceptibility to available MABs. However, the rate of failure in case of MABs was high even before the emergence of variants associated with their inefficacy and was present (although not statistically significant) even when treatments were compared in a subgroup of patients treated during the Omicron BA.1 period with sotrovimab, which is active against this variant. Therefore, several issues warrant consideration. First, some patients developed failure on the same day of infusion of MABs, raising a question if a possible worsening of respiratory function due to MAB administration could be the reason.33 Second, the selective pressure of monoclonal antibodies which persist in patient's system, in combination with the lack of an effective endogenous immune response, may promote the emergence of SARS-CoV-2 escape mutations. In a recent prospective study, the use of sotrovimab in immunocompromised patients was associated with prolonged viral shedding, and after longitudinal viral sequencing, the authors found that 32.6% (14/43) of immunocompromised patients who received sotrovimab developed spike protein mutations that substantially reduced susceptibility to sotrovimab in a neutralization assay, while patients who received combination treatment with remdesivir had a significant reduction in the selection of escape variants.34 Similar findings were noted in a previous study reporting that two of five immunocompromised patients treated with sotrovimab developed resistance.35 Finally, we cannot exclude that despite the predominance of certain variants, some patients might be infected with another variant, resistant to the MABs used. In fact, in a general population of patients during the Omicron BA.2 predominance, similar efficacy of bebtelovimab (active against all main Omicron subvariants) and nirmatrelvir/ritonavir was noted (1.4% and 1.2%), but such a low rate of progression, and low rate of severely immunocompromised patients might not allow detection of any differences applicable to subgroups such as the HM population.36 Whatever the reason for the lower efficacy of MABs observed in our cohort, considering a possible negative effect of emerging variants, antivirals seem to provide a more reliable treatment option.
Analysing specifically the predictors of failure during Omicron infection, the protective role of younger age and higher number of vaccine doses was confirmed, but an effect of the underlying disease emerged. The highest rate of failure was observed in case of AML/MDS, while HR was 30% lower in lymphoid disorders such as NHL/CLL and almost 80% lower for all other diseases. The negative outcome in AML/MDS patients might be related to the aggressiveness of their underlying disease and high intensity chemotherapy, and was present also in initial studies in HM patients as a risk factor for higher mortality.1, 37
COVID-19-related mortality was 3.4% in the whole population, but as high as 21% in the pre-Omicron period despite early treatment, and 2.3% during the Omicron period, with age and AML/MDS being other independent predictors. COVID-19-associated mortality and overall mortality were lower than in previous reports in HM patients (e.g., 18% in 3801 patients from a European survey), but still significantly higher than in the general population considered at risk for severe COVID-19 and thus deserving early treatment.4 Moreover, in our cohort, the overall mortality doubled from day 30 to day 90, confirming the previous observation from 936 solid-organ transplant recipients reporting delayed mortality in that population.8 This might be partially due to the indirect impact of COVID-19 on patients’ survival including delayed access to chemotherapy or higher risk of late complications. Interestingly, the early treatment seemed to change the previously observed timing of COVID-19 worsening, which typically occurred early during infection, while in this cohort, half of the failures occurred more than 10 days and up to 35 days from the onset of infection.
The optimal management of patients who progress to severe COVID-19 remains to be established but highly effective strategies are required, since the mortality in those who progressed to severe COVID-19 remains very high (57% in the pre-Omicron and 26% in the Omicron period) despite provided treatment in accordance with current guidelines.16, 25 The difficulty in managing these patients also stems from the uncertainty which component — viral or inflammatory — is predominantly responsible for clinical worsening, and which treatment strategies might be most effective.
Prolonged shedding of SARS-CoV-2 virus is frequent in HM patients, and this study confirmed that even in case of early treatment during the Omicron period, 12% were still SARS-CoV-2-positive beyond day 30, which is almost identical to what was previously reported.22 Among the predictors of prolonged shedding, we confirmed the predisposing role of NHL/CLL, male sex and comorbidities.22, 38, 39 In addition, MABs were also associated with longer shedding, while the role of the Omicron variant and anti-CD20 treatment was not confirmed in multivariate analysis. The possible reasons for longer shedding in case of MABs might be lower efficacy against the Omicron variants and higher probability of inducing resistance due to prolonged exposure to MABs remaining in the patient's system.34, 35 The concerns regarding persistent SARS-CoV-2 infection include the risk of clinical relapse, limited access to healthcare and delay of chemotherapy and the threat of intrahost viral evolution. Viral dissemination and accelerated evolution, which may be responsible for immune escape from the initial infection, have been described in immunosuppressed patients with persistent or severe SARS-CoV-2 infection.21, 40, 41
Limitations of this study include retrospective design and change over time in circulating variants overlapping with the change in early treatment and increase in administered vaccine doses, making it difficult to ascertain the independent role of one factor versus another. Additionally, data on infecting variants and posttreatment sequencing were not routinely available. However, inclusion of the whole cohort provided a complete overview of over 300 consecutive HM patients diagnosed with SARS-CoV-2 infection during the time when early treatment was available and promptly administered. In addition, analyses of the Omicron-limited subgroup offered more updated information, while not excluding the pre-Omicron period showed how a change in the predominant variant can rapidly increase the prevalence of SARS-CoV-2 infection also in HM setting.
In conclusion, patients with HM are still at high risk for negative outcomes in case of SARS-CoV-2 infection, with 7.8% rate of progression to severe COVID-19 despite early treatment, almost universal vaccination and the Omicron variant. Moreover, those who experience disease progression continue to be at high risk for mortality. Our findings strongly support the recommendations to vaccinate with boosters and provide effective early treatment, keeping in mind that the efficacy of antivirals is currently not affected by the emergence of new variants.16
AUTHOR CONTRIBUTIONS
Malgorzata Mikulska, Chiara Sepulcri, Diletta Testi, Chiara Russo and Michele Bartoletti performed research and wrote the manuscript; Elisa Balletto, Linda Bussini, Chiara Dentone, Federica Magne, Sílvia Policarpo, Caterina Campoli, Federica Magne, Chiara Ghiggi, Alessandro Cilli, Sara Aquino, Chiara Ghiggi performed data collection; Malgorzata Mikulska, Maddalena Giannella, Daniele Roberto Giacobbe, Michele Bartoletti performed data analysis; Anna Maria Raiola, Francesca Bonifazi, Pierluigi Zinzani, Michele Cavo, Roberto Lemoli, Emanuele Angelucci, Pierluigi Viale, and Matteo Bassetti supervised the study. All Authors reviewed and approved the manuscript.
ACKNOWLEDGEMENTS
Open Access Funding provided by Universita degli Studi di Genova within the CRUI-CARE Agreement.
FUNDING INFORMATION
No funding was provided for this study.
CONFLICT OF INTEREST STATEMENT
Emanuele Angelucci reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Vertex Pharmaceuticals Incorporated and Celgene (BSM), Vifor Pharma, Novartis, Blue Bird Bio, Menarini Stemline, Glaxo, Regeneron, GILEAD and Novartis. Matteo Bassetti reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Bayer, BioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. The other authors have no conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




