Isocitrate dehydrogenase inhibitors as a bridge to allogeneic stem cell transplant in relapsed or refractory acute myeloid leukaemia
To the Editor,
One of the few curative options for refractory or relapsed acute myeloid leukaemia (AML) is allogeneic stem cell transplantation (allo-SCT).1 In patients eligible for allo-SCT, achieving a new complete remission (CR) is an important prerequisite.2 There is no consensus regarding the salvage regimen.1, 3 The recent development of several targeted therapies4 could allow the emergence of alternatives. Approximately 20% of patients have a somatic mutation in the enzyme isocitrate dehydrogenase (IDH) 1 or 2,5 an enzyme involved in leukemogenesis through the disruption of cell signalling and epigenetic dysregulation.6 In relapsed or refractory AML, ivosidenib (which inhibits the IDH1 enzyme) and enasidenib (which inhibits IDH2) have shown efficacy with a response rate of approximately 30%–40% in monotherapy.7, 8 However, these responses appear to be transient and do not necessarily translate into survival benefit as illustrated by the IDHENTIFY phase 3 trial (NCT02577406) which failed to show an improvement in overall survival (OS) in relapsed or refractory IDH2-mutated AML.9 Few data are available regarding the use of IDHi as a bridge to allo-SCT. Recently, DiNardo et al.10 reported the use of the IDH1 inhibitor ivosidenib in the subgroup of patients enrolled in the phase 1 trial that proceeded to allo-SCT (n = 17). Patients not eligible for an intensive salvage regimen showed a favourable outcome after allo-SCT. No similar studies have been performed in patients receiving an IDH2 inhibitor or in patients deemed equally eligible for intensive treatment.
We report the use of IDHi in a real-life setting in patients with refractory or relapsed AML considered eligible for allo-SCT. This is a single-centre retrospective study of patients treated in our haematology department at Saint-Antoine Hospital between January 2019 and March 2021. We consecutively included all patients with relapsed or refractory AML with IDH1 or IDH2 mutation in whom allo-SCT seemed possible at the time of IDHi initiation. The department physicians assessed allo-SCT eligibility. The study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee. Results are reported on an intention-to-treat basis. Mutational analysis was performed by next-generation sequencing (NGS) targeted resequencing of 41 genes or a 64-genes panel, as already described.11 Patient were treated with oral ivosidenib 500 mg daily (QD) or enasidenib 100 mg QD. The treatment continued until the day before the start of the conditioning regimen for those undergoing transplantation (see Data S1 for detailed study design).
Eleven patients with relapsed or refractory AML and IDH1 or IDH2 mutation eligible for allo-SCT were included in this study. Their characteristics are shown in Table 1. The median age for the entire cohort was 60.6 years (range, 29.3–63.7). Seven patients had an IDH2 mutation, and four patients had an IDH1 mutation. Six patients had relapsed while five patients failed to achieve CR/CRi following at least one cycle of intensive chemotherapy. Patients received a median of one line of treatment (range, 1–4) prior to IDHi therapy and three patients had already received a first allo-SCT. In addition to IDH mutations, patients harboured a median number of four co-occurring mutations (range, 2–10) including Fms-like tyrosine kinase 3 mutation in one patient (Data S1). At the initiation of the IDHi treatment, patient 8 was intubated and so enasidenib was administered via a nasogastric tube during the first 15 days of treatment (Data S1).
| Enasidenib 100 mg QD before alloSCT (n = 7) | Ivosidenib 500 mg QD before alloSCT (n = 4) | All patients (n = 11) | |
|---|---|---|---|
| Age, median, years (range) | 60.6 (56.9–63.6) | 56.2 (29.3–63.7) | 60.6 (29.3–63.7) |
| Female/male, n (%) | 0/7 | 1/3 | 1/10 (9) |
| Prior history of MDS/MPN, n (%) | 3 | 1 | 4 (36.4) |
| ELN2017 risk status, n (%) | |||
| Favourable | 1 | 0 | 1 (9.1) |
| Intermediate | 3 | 3 | 6 (54.5) |
| Adverse | 3 | 1 | 4 (36.4) |
| IDH mutation, n (%) | |||
| IDH1 | 0 | 4 | |
| IDH2 | 7 | 0 | |
| Type of IDH mutation | |||
| IDH2/R140 | 7 | ||
| IDH2/R172 | 1 | ||
| IDH1/R132 | 4 | ||
| Number of lines before IDHi, n (range) | 1 (1–2) | 1.5 (1–4) | 1 (1–4) |
| Status before IDHi n (%) | |||
| Relapsed | 3 | 3 | 6 (54.5) |
| Refractory | 4 | 1 | 5 (45.5) |
| Prior allo-SCT | 1 | 2 | 3 (27.3) |
| Best response to treatment, n (%) | |||
| ORR (CR+CRi) | 4 | 3 | 7 (63.6) |
| CR | 3 | 3 | 6 (54.5) |
| CRi | 1 | 0 | 1 (9.1) |
| Progressive disease | 2 | 1 | 3 (27.3) |
| Stable disease | 1 | 0 | 1 (9.1) |
| Time to CR, days (range) 30–330 | 58.5 (45–330) | 73 (30–88) | 60 (30–330) |
| Duration of treatment, days (range) | 72 (13–812) | 111 (92–126) | 101 (13–812) |
| Patients proceeding to allo-SCT, n (%) | 4 | 3 | 7 (63.6) |
| Relapse or progressive disease, n (%) | 2 | 1 | 3 (27.3) |
| Deaths, n (%) | 1 | 1 | 2 (18.2) |
| Median follow-up since diagnosis, months (range) | 24.7 (15.2–36.2) | 46.3 (14.6–47 | 24.7 (14.6–57.8) |
| Median follow-up since allo-SCT, months (range) | 16.0 (13.7–31.7) | 16.5 (4.5–28) | 16.5 (4.5–31.7) |
| Three-year overall survival (%, CI95) | 85.7 (33.4–97.9) | ||
- Abbreviations: allo-SCT, allogeneic stem cell transplantation; CI95, 95% confidence interval; CR, complete remission; CRi, complete remission with incomplete count recovery; IDH, isocitrate dehydrogenase; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; ORR, overall response rate; QD, daily.
No treatment was stopped due to adverse events. There were no treatment-related deaths (one patient, treated with ivosidenib, developed a differentiation syndrome which evolved favourably after appropriate treatment and three patients developed a grade III–IV infection during IDHi treatment). All patients received IDHi monotherapy except patient 8 who received enasidenib and gemtuzumab ozogamicin. Of the whole cohort, seven (63.6%) patients showed a CR or CR with incomplete count recovery (CRi) to IDHi treatment: three out of four patients with ivosidenib (IDH1 mutated) and four out of seven with enasidenib (IDH2 mutated). The median response time was 60 days (range, 30–330). The median duration of the treatment was 101 days (range, 13–812). None of the responding patients achieved complete molecular remission as assessed by NGS during IDHi treatment. Seven (63.6%) patients were able to undergo allo-SCT after treatment with IDHi: three patients out of four in the IDH1 group and four out of seven in the IDH2 group. Among these seven patients, four were in CR or CRi at the time of allo-SCT. Five patients had sequential conditioning (chemotherapy with broad antitumour activity followed by reduced-intensity conditioning regimen) and two patients had reduced-intensity conditioning.12 All patients had a haploidentical donor. All patients that proceeded to allo-SCT are still in CR and the IDH mutation is no longer detectable by NGS analysis (response for two patients is shown in Figure 1B,C). No patients received IDHi as maintenance therapy after allo-SCT; four patients received a prophylactic treatment with azacitidine.
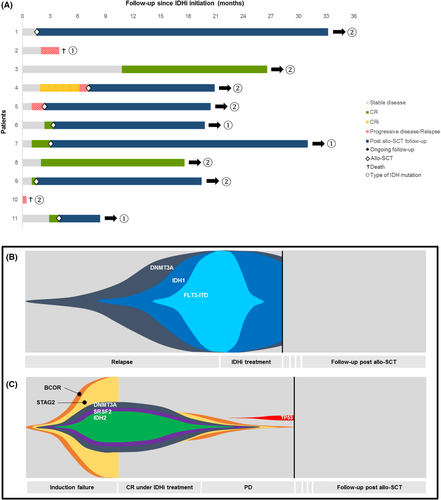
With a median follow-up since diagnosis of 24.7 months (range, 14.6–57.8), nine of the 11 (81.8%) patients are alive including all seven patients that proceeded to allo-SCT after IDHi. Median OS was not reached. The two-year and three-year OS was 100% and 85.7%, respectively. Of the four patients who did not proceed to allo-SCT: two patients progressed while receiving IDHi, one patient was deemed ineligible while in CR and is still in CR under IDHi (follow-up of 17.6 months; this patient harbours both an NPM1 and an IDH2 mutation). One patient refused the allo-SCT while in CR and he is still in CR.
We observed a high response rate with 54.5% CR in the whole cohort. In comparison, when using our gemtuzumab ozogamicin-based intensive salvage regimen, we observed a CR rate of 67% and 54% for IDH mutated patients.13 The use of IDHi compares favourably with what can be observed with an intensive salvage regimen, as there were no deaths from infection or adverse events. The responses were nevertheless shallow with no patient achieving a complete molecular response: all patients harboured an IDH mutation before allo-SCT. As shown by DiNardo et al.,10 this does not seem to influence the outcome of patients after allo-SCT including IDH2 patients. Indeed, for all patients who proceeded to allo-SCT, their IDH mutation was no longer detectable by NGS analysis. On an intention-to-treat basis, 63.6% of patients were able to benefit from allo-SCT. This rate is quite satisfactory compared to other strategies.14
In conclusion, the use of IDHi as a bridge to allo-SCT in patients with relapsed or refractory AML with IDH1 or IDH2 mutation is an effective strategy. Clearance of the IDH1 or IDH2 mutation does not appear to influence the outcome of the patient after allo-SCT.
AUTHOR CONTRIBUTIONS
Alexis Genthon designed the study, performed the study, analysed the data, and drafted the manuscript. Diana Dragoi performed the research and revised the manuscript. Mara Memoli, Pierre Hirsch, Fabrizia Favale, Ludovic Suner, Michael Chaquin, Pierre Boncoeur, Zora Marjanovic, Agnes Bonnin, Simona Sestili, Remy Dulery, Florent Malard, Eolia Brissot, Anne Banet, Zoe van de Wyngaert, Anne Vekhoff, Francois Delhommeau, Mohamad Mohty and Ollivier Legrand provided critical review. All authors approved the final version of the manuscript.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
Alexis Genthon has received honoraria from Abbvie. Mohamad Mohty has received grants/research supports from Sanofi, Janssen, and honoraria or consultation fees: fromJazz Pharma, Amgen, Janssen, Celgene, Takeda, Novartis, GSK, Adaptive, Pfizer, Astellas, Gilead, Oncopeptides and Sanofi. The other authors have no conflict of interest.
PATIENT CONSENT STATEMENT
Research was conducted in accordance with local and international guidelines. The investigators have informed the patients about the research in compliance with the methodology “MR-004” of the French Data Commission.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Our article does not include data from third parties.
Open Research
DATA AVAILABILITY STATEMENT
The de-identified data that support the findings of this study are available on request from the corresponding author.