Managing argatroban in heparin-induced thrombocytopenia: A retrospective analysis of 729 treatment days in 32 patients with confirmed heparin-induced thrombocytopenia
Summary
Argatroban is a first-line anticoagulant for patients with heparin-induced thrombocytopenia (HIT). Published data on practical aspects of its use in HIT are lacking. We aim to establish recommendations based on our experience. This cohort of 32 patients is the largest describing cases of HIT confirmed by a functional assay treated with argatroban. Among patients with normal liver function, median starting argatroban doses (SAD) of 0.54, 0.98, and 1.27 μg/kg/min reached steady-state plasmatic argatroban concentrations (PAC) of 0–0.39, 0.40–0.99, and 1.00–1.5 μg/ml, respectively. Median argatroban dose increases (ADI) induced similar median steady-state PAC increases (Δ μg/kg/min ≈ Δ μg/ml). PAC measurements performed more than 240 min after SAD or ADI were significantly higher compared to earlier controls. Quantitative PAC measurements and thrombin time (TT) appeared adequate for monitoring. Thirty-eight percent of the thrombotic events were preceded by PAC below 0.4 μg/ml. Four hours after argatroban discontinuation, median international normalised ratio (INR) decrease was −1.2. We suggest: (i) monitoring argatroban with PAC or TT at least 240 min after SAD and/or AID; (ii) using SAD of 1.0 μg/kg/min and ADI of at least 0.2 μg/kg/min when liver function is normal; (iii) targeting therapeutic PAC of 0.5–1.0 μg/ml; and (iv) targeting INR of 3.5–4.5 when bridging argatroban with vitamin K antagonists.
INTRODUCTION
Heparin-induced thrombocytopenia (HIT) is an adverse effect of unfractionated (UFH) and low-molecular-weight heparin (LMWH), occurring with variable risk depending on the type and dose of heparin and the clinical context.1-4 HIT is characterised by a severe limb- and life-threatening pro-thrombotic state requiring immediate cessation of all heparin and a switch to an alternative non-heparin anticoagulant.5-9
Argatroban is an effective first-line therapy for HIT5, 10 and was described in a recent meta-analysis as being superior to other parenteral drugs used for the management of HIT.11 Argatroban is a synthetic, reversible and competitive direct thrombin inhibitor, which binds thrombin on its catalytic site12 and is hepatically metabolised.13
Less is known, however, about several practical aspects of anticoagulation with argatroban. The magnitude of starting doses and dose adaptations, as well as the degree of dose reduction required among patients with different types of hepatic dysfunction remain unclear and are performed empirically. Furthermore, despite recent guidelines,10 the best timing of, and laboratory assays for, argatroban monitoring still remain open to discussion. The aim of our study was to investigate how argatroban therapy is conducted at our institution and to develop evidence-based in-house recommendations for an optimal anticoagulation with argatroban in patients with HIT.
METHODS
Study design
This was a retrospective observational derivation study. Data were collected between October 2018 and February 2019 and analysed between February 2019 and August 2021.
Patient cohort
Between August 2014 and February 2019, 32 patients with confirmed HIT14 [positive anti-PF4/heparin antibodies by HemosIL-Acustar-HIT-IgG (Instrumentation Laboratory GmbH, Munich, Germany) (a chemiluminescent immunoassay, CLIA) and ID-H/PF4-PaGIA (Bio-Rad, DiaMed SA, Basel, Switzerland) (a particle gel immunoassay, PaGIA) and positive heparin-induced platelet aggregation (HIPA, University Hospital, Greifswald, Germany)] received argatroban at our institution and were included in this study.
Laboratory monitoring of argatroban
We performed the following assays on an automated coagulometer (Sysmex CS-5100; Siemens Healthineers, Erlangen, Germany): (i) activated partial thromboplastin time (aPTT; Pathromtin SL®; Siemens Healthineers, Erlangen, Germany); (ii) thrombin time (TT; Thromboclotin®, Siemens Healthineers, Erlangen, Germany) with a final thrombin concentration of 1.25 U/ml; and (iii) assessment of the plasma argatroban concentration by a commercial diluted TT assay (Hemoclot Thrombin Inhibitors; Hyphen Biomed, Neuville-sur-Oise, France).
Definition of patients with impaired hepatic function
As argatroban is mainly metabolised in the liver, we divided the studied patients into four categories according to their estimated liver function. (i) Normal liver function: serum alanine aminotransferase (ALT) levels less than 180 U/l [three times the upper limit of normal (ULN)] and serum total bilirubin levels less than 25.5 μmol/l.13 When a patient previously had ALT higher than 180 U/l and/or serum total bilirubin higher than 25.5 μmol/l and recovered, he was included into the ‘normal liver function group’ five days after recovery of ALT less than 180 U/l and serum total bilirubin less than 25.5 μmol/l. (ii) Liver cytolysis: ALT higher than 180 U/l and serum total bilirubin less than 25.5 μmol/l. (iii) Impaired bilirubin excretion: ALT less than 180 U/l and serum total bilirubin higher than 25.5 μmol/l. (iv) Liver cytolysis associated with impaired bilirubin excretion: ALT higher than 180 U/l and serum total bilirubin higher than 25.5 μmol/l.
Statistics
Median and interquartile range (IQR) values were calculated with Excel (Microsoft Schweiz GmbH, Zürich-Flughafen, Switzerland). We employed MedCalc (version 15.11.0; MedCalc Software Ltd, Ostend, Belgium) to perform Passing–Bablock regression analysis. Sigma Plot (version 13.0; Systat Software GmbH, Erkrath, Germany) was used to create Figure 1 and GraphPad Prism (version 8; GraphPad Software, San Diego, USA) was used to create Figures 2-8 and to perform Mann–Whitney tests (t-test, unpaired values, non-parametric distribution), Wilcoxon tests (t-test, paired values, non-parametric distribution) and Spearmann correlation measures.
Ethics
The study complies with the guidelines of the Institutional Ethical Board (Commission Cantonale Vaudoise d'Ethique de la Recherche sur l'Être Humain, CER-VD, protocol number 497/95) and was accepted for quality control assessment of laboratory and clinical management practice.
RESULTS
Patient population
Thirty-two patients with confirmed HIT (15 of them presenting with HIT and thrombosis, HIT-T) were treated with argatroban. Twenty-seven patients (84%) only received argatroban and five (16%) received multiple treatments (Table 1). Overall, we studied 729 argatroban treatment days. Median duration of argatroban therapy was 17.5 days (IQR 10–29.5).
| Feature | |
|---|---|
| Females, % (n/N) | 47 (15/32) |
| Age, years; median (IQR; range) | 67.4 (58.3–75.1; 33.5–88.9) |
| Hospital ward | |
| Cardiac surgery | 11a |
| Vascular surgery | 6a |
| Orthopaedics and traumatology surgery | 0 |
| Thoracic surgery | 4 |
| Neurosurgery | 1 |
| Intensive care unit | 4 |
| Internal medicine | 2 |
| Cardiology | 5 |
| Heparin treatment and regimen | |
| LMWH prophylactic | 9 |
| LMWH therapeutic | 4 |
| UFH prophylactic | 25 |
| UFH therapeutic | 20 |
| HIT diagnosis features | |
| 4 T score; median (IQR; range) | 5 (5–5; 3–7) |
| CLIA, U/ml; median (IQR; range) | 8.64 (1.96–21.69; 0.15–129) |
| PaGIA, titre; median (IQR; range) | 8 (8–32; 2–64) |
| HIPA, number of positive | 32 |
| Type of HIT | |
| HIT without thrombosis, n (%) | 17 (53) |
| HIT-T, n (%) | 15 (47) |
| Treatments | |
| Argatroban only | 27 |
| Danaparoid prior to argatroban | 3 (UPN 17.173, UPN 17.079, UPN 15.103) |
| IVIG | 3 (UPN 17.173, UPN 17.042b, UPN 17.222) |
| Plasmapheresis | 1 (UPN 17.042b) |
| Argatroban treatment, days | 729 |
| Median duration (days) | 17.5 |
| IQR duration (days) | 10; 29.5 |
| Range duration (days, min; max) | 3; 109 |
| Complications under argatroban | |
| Arterial thromboembolic events, n (%) | 6 (19) |
| Venous thromboembolic events, n (%) | 7 (25) |
| Bleeding events, minor, n (%) | 1 (3) |
| Bleeding events, major, n (%) | 4 (13) |
| Outcomes | See also Tables 2–4 |
| Limb amputation, n (%) | 4 (13) |
| In-hospital mortality, n (%) | 4 (13) |
- Abbreviations: CLIA, chemiluminescent immunoassay; HIPA, heparin-induced platelet aggregation; HIT, heparin-induced thrombocytopenia; IQR, interquartile range; IVIG, intravenous immunoglobulin; LMWH, low-molecular-weight heparin; PaGIA, particle gel immunoassay; UFH, unfractionated heparin.
- a One patient underwent two operations (cardiac and vascular surgery) on the same day.
- b One patient received plasmapheresis and then IVIG after argatroban discontinuation.
Complications
Compared to the cohort published by Tardy-Poncet et al.,15 we registered a higher rate of death related to HIT (n = 1/32 vs. 0/16), a similar rate of thromboembolic complications (n = 13/32, 41% vs. n = 7/16, 44%), and less major bleeding (n = 4/32, 13% vs. n = 3/16, 19%) and overall death rates (n = 4/32, 13% vs. n = 4/16, 25%). Individual clinical courses are detailed in Table 2.
| Sex | Age | Care unit; underlying main disease(s) and management at our institution | Type of heparin and dose | Platelet count nadir value (G/l) | 4T score | HIT versus HIT-T | HIT-T: Type of thrombo-embolic event at diagnosis | Diagnostic work-up | CLIA (U/ml) | PaGIA (titre) | Type of treatment and duration (days) | Thrombo-embolic events during treatment YES/NO *see Table 3 | Bleeding events YES/NO *see Table 4 | Limb outcome: Amputation and day of Argatroban treatment | Final outcome: Discharge xxx days after diagnosis of HIT versus hospital transfer | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. UPN: 18.130 | F | 63 | Thoracic surgery; idiopathic pulmonary fibrosis and bilateral lung transplant | Prophylactic UFH, then prophylactic LMWH | 51 | 5 | HIT-T | Pulmonary embolism and internal jugular vein thrombosis (secondary to catheter & HIT) | Chest CT scan with contrast product ultrasonography by an angiologist | 7.03 | 8 |
Argatroban; 11 days Central venous catheter (jugular) already removed at time of HIT diagnosis |
No | No | No amputation | Hospital transfer 11 days after diagnosis of HIT |
| 2. UPN: 18.022 | F | 52 | Cardiac surgery; pheochromocytoma and right adrenalectomy vena cava and right atrium thrombi thrombectomies | Therapeutic UFH, then therapeutic LMWH | 111 | 5 | HIT | None | N/A | 11.70 | 16 | Argatroban; 10 days | YES: 1* (SVT LLL) | NO | No amputation | Discharged 14 days after diagnosis of HIT |
| 3. UPN: 17.234 | M | 69 | Cardiac and vascular surgery; ischaemic heart disease with triple coronary bypass; left carotid stenosis with carotid endarterectomy (two simultaneous operations) | Prophylactic UFH, then prophylactic LMWH | 48 | 4 | HIT | None | N/A | 28.00 | >16 | Argatroban; 18 days | NO | NO | No amputation | Discharged 24 days after diagnosis of HIT |
| 4. UPN: 17.222 | M | 72 | Cardiac surgery; severe aortic valve stenosis with bioprothetic aortic replacement valve | Prophylactic UFH, then prophylactic LMWH | 18 | 5 | HIT | None | N/A | 12.56 | >16 | Argatroban; 18 days IVIG | NO | YES; 1* (minor bleeding; spontaneous haematoms on the arms and forearms)* | No amputation | Hospital transfer 18 days after diagnosis of HIT |
| 5. UPN: 17.206 | M | 89 | Cardiac surgery; ischaemic heart disease with triple coronary bypass; severe aortic valve stenosis and bioprothetic aortic replacement valve | Prophylactic UFH, then prophylactic LMWH, then prophylactic UFH | 54 | 5 | HIT-T | Fibular artery thrombosis | Ultrasonography and plethysmography by an angiologist | 70.88 | 16 | Argatroban; 14 days | NO | NO | No amputation | Discharged 23 days after diagnosis of HIT |
| 6. UPN: 17.173 | F | 29 | Internal medicine; systemic lupus erythematosus with antiphospholipid antibodies syndrome and heparin anticoagulation | Prophylactic UFH, then therapeutic UFH, then therapeutic LMWH | 5 | 5 | HIT | None | N/A | 1.40 | 16 | Danaparoid; 2 days then argatroban; 19 days IVIG | NO | NO | No amputation | Discharged 22 days after diagnosis of HIT |
| 7. UPN: 17.081 | F | 75 | Cardiology; acute heart failure and anticoagulation for mechanical mitral valve | Therapeutic UFH, then prophylactic UFH | 79 | 4 | HIT | None | N/A | 10.24 | 8 | Argatroban; 24 days | NO | NO | No amputation | Discharged 32 days after HIT diagnosis |
| 8. UPN: 17.079 | M | 68 | Intensive care unit; septic shock secondary to Balthazar E pancreatitis | Prophylactic UFH | 16 | 4 | HIT | None | N/A | 12.42 | >32 | Danaparoid; 8 days argatroban; 47 days | NO | NO | No amputation | Discharged 104 days after HIT diagnosis |
| 9. UPN: 18.148 | M | 89 | Vascular surgery; bilateral advanced arterial peripheral artery disease with two arterio-arterial bypasses, one Fogarty thrombo-embolectomy and one thrombolysis | Therapeutic UFH | 49 | 6 | HIT-T | External illiac artery and commune femoral artery thrombosis | Ultrasonography and plethysmography by an angiologist; arterial CT scan with contrast product | >128 | 8 | Argatroban; 44 days; prosthetic bypass confection; lower left limb | YES: 4* (1× arterial prosthetic bypass: 1× PE 1× DVT 1× arterial; lower left proximal limb) | NO | Gritti amputation of right lower limb 16 days after HIT diagnosis | Deceased 44 days after HIT diagnosis (pneumonia with cardiac failure) |
| 10. UPN: 17.042 | M | 75 | Cardiology; ischaemic heart disease with cardiogenic shock and angioplasty with stenting | Therapeutic and then prophylactic UFH | 30 | 4 | HIT-T | Internal jugular vein thrombosis (secondary to catheter & HIT) | Ultrasonography by an angiologist | 2.14 | 004 | Argatroban; 28 days, IVIG plasmapheresis because of discontinuation of argatroban (haemorragic shock) | NO | YES; 1* (major bleeding; haemorragic shock after self-snatch of urinary catheter in the context of confusional state) | No amputation | Discharged 69 days after HIT diagnosis |
| 11. UPN: 16.269 | F | 73 | Cardiac surgery; ischaemic heart disease, ascendant aorta aneurysm with moderate aortic valve regurgitation. Triple coronary bypass, bioprothetic aortic replacement valve and ascendant aorta replacement | Therapeutic and then prophylactic UFH | 62 | 5 | HIT | None | N/A | 2.98 | N.A. | Argatroban; 10 days | NO | NO | No amputation | Discharged 13 days after HIT diagnosis |
| 12. UPN: 16.193 | M | 64 | Thoracic surgery; mediastinal sarcoma with vena cava superior infiltration and tumour resection | Prophylactic UFH, then therapeutic UFH and then prophylactic LMWH | 75 | 5 | HIT-T | Bilateral pulmonary embolism | Chest CT scan with contrast product | 2.05 | >32 | Argatroban; 3 days | YES: 1* (internal carotid artery thrombosis and stroke) | YES: 1* (intracranial bleeding secondary to ischaemic stroke) | No amputation | Deceased 3 days after diagnosis of HIT (massive PE, pneumonia, intracranial bleeding with cerebral herniation) |
| 13. UPN: 16.199 | M | 59 | Thoracic surgery; empyema and thoracoscopy with pulmonary decortication | Prophylactic LMWH | 134 | 5 | HIT-T | Pulmonary embolism | Chest CT scan with contrast product | 20.71 | >32 | Argatroban; 8 days | NO | NO | No amputation | Discharged 8 days after HIT diagnosis |
| 14. UPN: 16.151 | M | 81 | Cardiac surgery; severe aortic valve stenosis and bioprothetic aortic replacement valve | Therapeutic LMWH and then prophylactic UFH | 71 | 5 | HIT | None | N/A | 1.70 | 8 | Argatroban; 10 days | NO | NO | No amputation | Discharged 15 days after HIT diagnosis |
| 15. UPN: 16.225 | M | 71 | Vascular surgery; abdominal aorta aneurysm and vascular endoprothesis | Prophylactic UFH | 32 | 5 | HIT | None | N/A | >128 | >32 | Argatroban; 29 days | NO | NO | No amputation | Discharged 31 days after HIT diagnosis |
| 16. UPN:16.351 | F | 48 | Intensive care unit; second-degree burns on 14% of body surface area, including sub-glottic burn | Prophylactic LMWH and then therapeutic UFH (HIT-T) | 79 | 6 | HIT-T | Deep vein and superficial vein thrombosis | Ultrasonography by an angiologist | 1.09 | >16 | Argatroban; 13 days | NO | NO | No amputation | Discharged 14 days after HIT diagnosis |
| 17. UPN: 17.201 | F | 67 | Cardiac surgery; ascendant and descendant thoracic, supra-renal abdominal aortic ecstasia with prosthetic replacement of ascending aorta and thoracic aorta endoprothesis | Prophylactic UFH | 25 | 5 | HIT | None | N/A | 1.00 | 2 | Argatroban; 49 days | NO | NO | No amputation | Discharged 93 days after HIT diagnosis |
| 18. UPN: 16.305 | M | 50 | Cardiology; anterior STEMI with angioplasty and stenting | Prophylactic, then therapeutic, then prophylactic UFH | 56 | 7 | HIT-T | Bilateral pulmonary embolism and bilateral deep vein thrombosis | Chest CT scan with contrast product ultrasonography by an angiologist | 33.15 | >16 | Argatroban; 31 days | NO | NO | No amputation | Discharged 79 days after HIT diagnosis |
| 19. UPN: 15.103 | F | 53 | Intensive care unit; perimyocarditis with cardiogenic shock, septic shock and disseminated intravascular coagulation with heparin anticoagulation | Prophylactic, then therapeutic UFH | 19 | 5 | HIT | None | N/A | 0.74 | 8 | Danaparoid; 6 days and then argatroban; 109 days | NO | NO |
1: Left lower limb Gritti amputation 13 days after HIT diagnosis 2: Right heel necrosectomy 43 days after HIT diagnosis 3: Left fingers 2,3,4,5 amputations 50 days after HIT diagnosis 4: Right fingers 2,3,4 amputation and right foot toes 1,3,4,5 amputation 104 days after HIT diagnosis NB: patient suffered acute hepatic necrosis and ischaemic limb necrosis before onset of HIT |
Discharged 155 days after HIT diagnosis |
| 20. UPN: 14.038 | M | 61 | Thoracic surgery; metastatic neuro-endocrine tumour and exploratory thoracotomy with oncologic surgery | Prophylactic, then therapeutic UFH | 25 | 4 | HIT | None | thrombin time N/A | 0.15 | 4 | Argatroban; 17 days | YES: 1* (bilateral PE) | NO | No amputation | Discharged 28 days after HIT diagnosis |
| 21. UPN: 14.049 | F | 78 | Internal medicine; metastatic small-cell lung carcinoma and chemotherapy | Port-a-cath flushing with UFH, then therapeutic LMWH, then therapeutic UFH | 23 | 4 | HIT-T | Pulmonary embolism and internal jugular and subclavian veins thrombosis | Chest CT scan with contrast product ultrasonography by an angiologist | 11.84 | 32 | Argatroban; 8 days | Check; YES? | NO | No amputation | Discharged 8 days after HIT diagnosis |
| 22. UPN: 14.072 | F | 83 | Neurosurgery; glioblastoma and radiochemotherapy | Prophylactic UFH | 63 | 5 | HIT-T | Multiple pulmonary embolisms | Chest CT scan with contrast product | 14.16 | 32 |
Cava filter on day −5 of treatment argatroban; 24 days |
YES: 1* (vena cava filter and bilateral pelvic veins thrombosis) | NO | No amputation | Deceased 46 days after HIT diagnosis |
| 23. UPN: 15.001 | M | 72 | Intensive care unit; metastatic prostate adenocarcinoma with pleural carcinosis and right thoracotomy with right pleural decortication | Prophylactic, then therapeutic UFH | 56 | 5 | HIT-T | Radial artery thrombosis (when radial catheter was removed) | Ultrasonography by an angiologist | 24.62 | 32 | Argatroban; 5 days | NO | NO | No amputation | Deceased 5 days after HIT diagnosis (metastatic prostatacarcinoma with pleural carcinosis and global respirator insufficiency) |
| 24. UPN: 15.138 | M | 60 | Vascular surgery; peripheral artery embolies with rapidly progressive lower limb ischaemia and lower limb bypass and thrombolysis | Therapeutic UFH | 63 | 7 | HIT-T | Aorto-iliac and infra-renal aorta aneurysm thrombosis | Ultrasonography by an angiologist; vascular CT scan with contrast product | 2.77 | 32 | Argatroban; 19 days | NO | NO | Gritti amputation of right lower limb 4 days after HIT diagnosis | Discharged 59 days after HIT diagnosis |
| 25. UPN: 15.143 | F | 86 | Cardiac surgery; ascendant aorta aneurysm and ascending aorta replacement | Prophylactic UFH, then prophylactic LMWH, then prophylactic UFH | 35 | 5 | HIT | None | N/A | 28.7 | 32 | Argatroban; 8 days | NO | NO | No amputation | Discharged 12 days after HIT diagnosis |
| 26. UPN: 15.160 | F | 66 | Vascular surgery; advanced bilateral peripheral artery disease and angioplasty with stenting, thrombolysis, Fogarty thrombectomy, bypass and endarterectomy | Prophylactic, then therapeutic UFH | 82 | 6 | HIT-T | Bilateral superficial femoral artery and venous popliteo-poplital bypass thrombosis | Ultrasonography by an angiologist | 1.37 | 8 |
Argatroban; 21 days Left lower limb prothetic bypass between superficial femoral artery and popliteal artery |
NO |
YES* 1 major bleeding; hematochezia (argatroban; ASS cardio and clopidogrel 1 minor bleeding; melena (argatroban and clopidogtrel) |
Burgess amputation of left lower limb 8 days after HIT diagnosis | Discharged 72 days after HIT diagnosis |
| 27. UPN: 15.166 | F | 54 | Cardiac surgery; severe aortic valve stenosis associated with ascendant aorta dilatation. Mechanic aortic replacement valve and ascending aorta replacement | Prophylactic, then therapeutic UFH | 14 | 6 | HIT | None | N/A | 17.97 | 8 | Argatroban; 16 days | NO | NO | No amputation | Discharged 17 days after HIT diagnosis |
| 28. UPN: 15.169 | M | 57 | Cardiac surgery; coronary heart disease and coronary bypass | Prophylactic LMWH | 23 | 6 | HIT-T | Splanchnic vein thrombosis with mesenteric ischaemia | Chest and abdominal CT with contrast product | 26.16 | 8 |
Argatroban; 21 days median laparotomy; resection of 1.5 m of bowel |
YES* thrombosis of principal left thumb artery | NO | No amputation resection of 1.5 m of ischaemic bowel 1 day after HIT diagnosis | Discharged 23 days after HIT diagnosis |
| 29. UPN: 15.186 | M | 33 | Cardiology; idiopathic dilatative cardiomyopathy with intra-ventricular thrombus and heparin anticoagulation | Therapeutic UFH | 55 | 5 | HIT | None | N/A | 3.55 | 8 | Argatroban; 18 days |
YES* Intra-ventricular thrombus (already present before HIT) with multiples embolic strokes; thrombo-emboly into the anterior Interventricular coronary artery |
YES; Major bleeding: intracranial bleeding secondary to ischaemic stroke | No amputation | Discharged 28 days after HIT diagnosis |
| 30. UPN: 18.225 | F | 65 | Cardiac surgery; severe aortic and moderate mitral valves stenosis; bioprothetic aortic and mitral replacement valves | Prophylactic UFH | 16 | 5 | HIT | None | N/A | 3.19 | 16 | Argatroban; 14 days | NO | NO | No amputation | Hospital transfer 19 days after HIT diagnosis (under AVK) |
| 31. UPN: 18.243 | M | 70 | Cardiology; heart failure with reduced ejection fraction and dilatative cardiomyopathy of unknown origin and left ventricular assist device | UFH therapeutic | 50 | 4 | HIT | None | N/A | 0.84 | 8 | Argatroban; 53 days | YES* Thrombosis of aortic valve leaflet (patient with impaired opening of the aortic valve; i.e. opening 1/10 cardiac cycles) | NO | No amputation | Discharged 109 days after HIT diagnosis |
| 32. UPN: 19.001 | F | 79 | Vascular surgery; advanced peripheral artery disease and thrombendareterectomy and Fogarty thromboembolectomy | Therapeutic, then prophylactic UFH | 38 | 3 | HIT-T | Arterial; common and external iliac arteries thrombosis | Ultrasonography by an angiologist | 5.59 | 4 | Argatroban; 10 days; left iliac arteries and left superficial and deep femoral arteries Fogarty thromboembolectomy; angioplasty and stenting of superficial femoral artery; short femoro-femoral bypass, angioplasty and stenting of left distal external iliac artery | NO | NO | No amputation | Hospital transfer 10 days after HIT diagnosis |
- Note: As per inclusion criteria, all 32 subjects were ‘HIPA-positive’. N/A, not applicable: no HIT-T.
- Abbreviations: ASA, acetylsalicylic acid; CLIA, chemiluminescent immunoassay; CT, computed tomography; DVT, deep vein thrombosis; F, female; HIPA, heparin-induced platelet aggregation; HIT, heparin-induced thrombocytopenia; HIT-T, HIT and thrombosis; IVIG, intravenous immunoglobulin; LMWH, low-molecular-weight heparin; M, male; PaGIA, particle gel immunoassay; PE, pulmonary embolism; STEMI, ST-segment elevation myocardial infarction; UFH, unfractionated heparin; UPN, unique patient number.
We observed 13 thromboembolic events among nine different patients (Table 3). Seven were venous and six were arterial thromboses. Five out of 13 (38%) thromboembolic events were related to an anticoagulation that was probably inadequate. Indeed, PAC had been below 0.4 μg/ml in the 24 h preceding diagnosis in four cases and three days before diagnosis in one patient. Additionally, other risk factors were present: recent surgery and immobilisation (n = 11), systemic infection (n = 8), active cancer (n = 6), body mass index (BMI) higher than 30 kg/m2 (n = 2), intravascular devices (n = 5), and/or poor haemodynamic conditions (n = 4).
| HIT type | Timing of the thromboembolic event; | Type and localisation | Argatroban dosage in the 24 h preceding the TE diagnosis; | PAC measured in the 24 h preceding the TE diagnosis; | Minimal PAC measured in the 24 h preceding the TE diagnosis (μg/ml) | Additional thrombotic risk factors | ||
|---|---|---|---|---|---|---|---|---|
| day of argatroban treatment | mean (detailed doses), μg/kg/min | mean (μg/ml) | ||||||
| 1. UPN: 18.022 | HIT | 4th | Deep vein thrombosis | 1.39 (1.33; 1.45) | 0.59 | 0.35b | High BMI; recent surgery; immobilisation; active cancer; systemic infection (urosepsis) | Multiple factors; possibly insufficient anticoagulationa |
| 2. UPN: 18.148 | HIT-T | 2nd | Femoro-iliac arterial prosthetic bypass thrombosis | 0.65 (1.0; 0.5; 0.45) | 1.04 | 0.91 | Recent surgery; immobilisation; systemic infection (pneumonia) | Multiple factors; possibly low blood flowe |
| 3. UPN: 18.148 | HIT-T | 28th | Bilateral lung emboli | 0.5 (0.5) | 0.45 | 0.43; NB: 0.26; there might be a diagnostic delay of the lung embolibc | Recent surgery; immobilisation; systemic infection (pneumonia) | Insufficient anticoagulationa |
| 4. UPN: 18.148 | HIT-T | 36th | Superficial femoral and popliteal arteries thrombosis | 0.6 (0.5; 0.59; 0.7) | 0.56 | 0.36 b | Recent surgery; immobilisation; systemic infection (pneumonia) | Low blood flow; possibly insufficient anticoagulationa |
| 5. UPN: 16.193 | HIT-T | 2nd | Internal carotid artery thrombosis and subsequent ischaemic stroke | 0.55 (0.5; 0.59) | 0.53 | 0.21b | High BMI; recent surgery; immobilisation; active cancer; systemic infection (pneumonia) | Multiple factors; possibly insufficient anticoagulationa |
| 6. UPN: 14.038 | HIT | 4th | Bilateral lung emboli | 0.37 (0.12; 0.39; 0.6) | 0.5 | 0.22b | Recent surgery; immobilisation; active cancer | Multiple factors; possibly insufficient anticoagulationa |
| 7. UPN: 14.049 | HIT-T | 4th | Subclavian vein and internal jugular vein thrombosis | 0.53 (0.45; 0.48; 0.5; 0.52; 0.69) | 0.20 | 0.11 | Active cancer; subclavian catheter for chemotherapy | Intravascular foreign materialf |
| 8. UPN: 14.072 | HIT-T | 8th | Vena cava filter thrombosis | 1.32 (1.21; 1.42) | 0.38 | 0.3 | Recent surgery immobilisation; active cancer | Intravascular foreign materialf |
| 9. UPN: 14.072 | HIT-T | 14th | Vena cava filter thrombosis extension and bilateral pelvic veins thrombosis | 1.86 (1.86) | 0.66 | 0.63 | Recent surgery; immobilisation; active cancer | Intravascular foreign materialf |
| 10. UPN: 15.169 | HIT-T | 2nd | Main thumb artery thrombosis | 0.61 (0.92; 0; 0.92) | 0.28 | 0 | Recent surgery; systemic infection secondary to mesenteric ischaemia; radial artery catheter | Intravascular foreign materialf |
| 11. UPN: 15.186 | HIT | 15th | Intra-cardiac thrombus with subsequent cardio-embolic occipital strokes | 2.29 (2.29) | 0.58 | 0.49 | Immobilisation; advanced heart failure (dilatative cardiomyopathy with intra-ventricular thrombus); coronary and ventricular multiple catheterisms | Intra-cardiac thrombus embolisation under adequate anticoagulationd |
| 12. UPN: 18.243 | HIT | 3rd | Left coronary leaflet of the aortic valve thrombosis | 0.27 (0.23; 0.29; 0.25; 0.29) | 0.50 | 0.37 | Recent surgery; immobilisation; systemic infection (pneumonia with sepsis); heart failure with reduced EF (because of an aortic valve briefly and partially opening 1/10 cardiac cycles) and ventricular dilatation; left jugular central venous catheter | Low blood flowd |
| 13. UPN: 18.243 | HIT | 27th | Internal jugular vein and subclavian vein thrombosis | 0.35 (0.35) | 0.75 | 0.65 | Recent surgery; immobilisation; systemic infection (pneumonia with sepsis); heart failure with reduced EF and ventricular dilatation; left jugular central venous catheter | Intravascular foreign materialf |
- Five thromboembolic events were related to a possibly insufficient anticoagulation (a). Among them, four events were preceded by a minimum PAC below 0.4 μg/ml (b) in the 24 h preceding the diagnosis. One event might have been diagnosed with delay, being preceded by a minimum PAC below 0.4 μg/ml three days before diagnosis confirmation (c). Three thromboembolic events were partially or fully related to poor haemodynamics; two events were associated with advanced cardiac failure with severely reduced ejection fraction (d) and one was associated with low blood flow through a prosthetic vascular bypass in a patient with advanced peripheral arterial disease (e). Five thromboembolic events were related to intravascular foreign material (f).
- Abbreviations: BMI, body mass index; EF, ejection fraction; HIT, heparin-induced thrombocytopenia; HIT-T, HIT and thrombosis; PAC, plasmatic argatroban concentrations; TE, thrombotic event; UPN, unique patient number.
- Bold indicate mean values.
Concerning the five bleeding events (Table 4), four were considered as major according to the International Society for Thrombosis and Haemostasis (ISTH) definitions.16, 17 PAC measured in the 24 h preceding these bleeding events were within target ranges in 4/5 patients. Among the major bleeding events, two intracranial haemorrages occurred after ischaemic strokes and were considered as secondary to anatomical brain lesions. The other two major bleeding events occurred under concomitant double antiplatelet therapy.
| HIT type | Timing of bleeding event; | Type and localisation | Blood products administered | Argatroban dosage in the 24 h preceding the TE diagnosis; | Mean PAC (μg/ml) measured in the preceding 24 h (day prior diagnosis and day of diagnosis of the bleeding event) | Maximal PAC (μg/ml) measured in the preceding 24 h-(day prior diagnosis and day of diagnosis of the bleeding event) | Additional bleeding risk factors (bleeding history—reduced haemoglobin—concomitant antiplatelet drug—renal impairment — anatomical lesions — recent surgery) | Suspected cause of bleeding events (excessive anticoagulation and antiaggregation versus anatomical lesion vs. undetermined) | |
|---|---|---|---|---|---|---|---|---|---|
| day of argatroban treatment | mean (detailed doses), μg/kg/min | ||||||||
| 1. UPN: 17.222 | HIT | 2nd | Minor bleeding; subcutaneous spontaneous haematoms on both upper limbs | 0 | 0.43 (0.25; 0.45; 0.58) | 0.23 | 0.33 | Recent aortic valve replacement; impaired renal function | Undetermined |
| 2. UPN: 17.042 | HIT-T | 2nd | Major bleeding; macrohematuria and jugular bleeding with haemorrhagic shockb | 3 units of packed red blood cells; 1 unit of fresh frozen plasma | 0.92 (0.92) | 0.55 | 0.77 |
Vesical, prostatic and ureteral lesions secondary to self-removal of urinary catheter; internal jugular vein lesion secondary to self-snatch of central venous catheter clopidogrel and aspirin treatments (recent stenting of RIVA); renal impairment; reduced haemoglobin |
Anatomical lesion; possibly ‘excessive’ antiaggregation |
| 3. UPN: 16.193 | HIT-T | 3rd | Major bleeding; intracerebral bleeding with cerebral herniationa | 0 | 0.59 (0.59) | 0.59 | 0.69 | Recent ischaemic stroke secondary to thrombosis of the right internal carotid artery | Anatomical lesion |
| 4. UPN: 15.160 | HIT-T | 9th | Major bleeding; hematochezia secondary to per-operative ischaemic colitisb |
2 units of packed red blood cells |
Mean: 0.39 (0; 0.41; 0.61; 0.52) |
0.26 | 0.45 |
Clopidogrel and aspirin treatments; reduced haemoglobin due to digestive angiodysplasia Ischaemic colitis |
Anatomical lesion and possibly ‘excessive’ anti-aggregation |
|
5 UPN: 15.186 |
HIT | 20th | Major bleeding; asymptomatic bilateral frontal intracerebral bleedings and left intracerebellar bleedinga | 0 | 2.29 (2.29) | 0.58 | 0.67 | Multiple cardio-embolic cerebral and cerebellar strokes | Anatomical lesion |
- Note: We observed five bleeding events, four of them being major and one of them being minor, according to the ISTH definition.16, 17 Mean and maximal PAC (μg/ml) measured in the 24 h preceding these five bleeding events seemed not to be excessive. Bold indicate mean values.
- Abbreviations: HIT, heparin-induced thrombocytopenia; PAC, plasmatic argatroban concentrations; RIVA, ramus interventricularis anterior; TE, thrombotic event; UPN, unique patient number.
- a Among the four major bleeding events, two were intracranial bleedings that occurred after ischaemic stroke.
- b The two other major bleeding events occurred under concomitant double antiplatelet therapy (aspirin and clopidogrel) and were related to anatomical lesions.
Outcomes
Four (13%) patients underwent limb amputation and four patients died (Table 2). Among patients that underwent amputation, three had established advanced peripheral arterial disease. The fourth patient suffered acute hepatic necrosis with ischaemic limb necrosis18 prior to HIT onset and underwent amputation during argatroban therapy (unique patient number, UPN 15.103). Among the four deceased patients, three were still under argatroban therapy at the time of death. In one patient (UPN 16.193), death was directly related to HIT. In two cases, advanced comorbidities may have contributed to the fatal outcome (UPN 18.148 and UPN 15.001).
Evolution of laboratory parameters under argatroban therapy
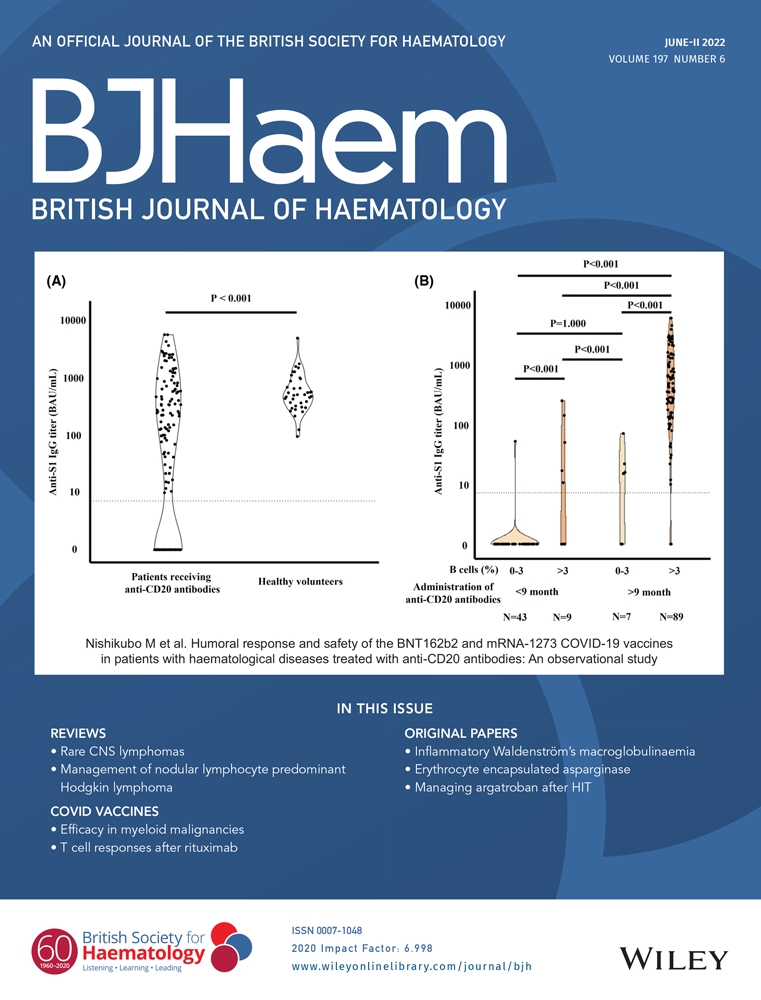
As depicted in Figure 1, we recorded a median platelet count nadir of 51 G/l on the second day of argatroban therapy, which was followed by full recovery (>150 G/l) on the seventh to eighth day of treatment. In parallel, median D-dimers levels progressively decreased.
Argatroban starting dose
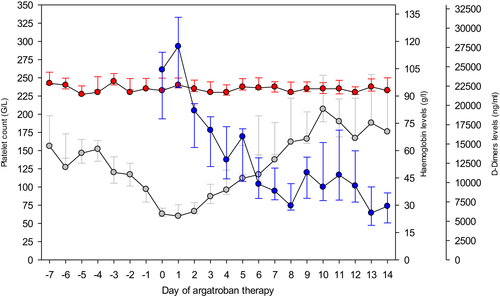
Every starting argatroban dose (SAD, μg/kg/min) and the corresponding PAC measurements (μg/ml) at time t′ (min) after SAD were analysed. Among patients with normal liver function, we divided all recorded SAD into two categories, according to an intended SAD of 0.5 or 1.0 μg/kg/min, respectively.
As illustrated in Figure 2A, after an overall median SAD of 0.5 μg/kg/min (IQR 0.48–0.58), median PAC among controls performed within 240 min reached 0.26 μg/ml (IQR 0.20–0.35) vs. 0.44 μg/ml (IQR 0.29–0.53) in controls at least 240 min after argatroban initiation (p-value = 0.0275). Of note, median SAD administered in patients with monitoring performed after at least 240 min (0.53 μg/kg/min) did not differ significantly from SAD applied in controls less than 240 min (0.50 μg/kg/min; p-value = 0.1594).
Figure 2B shows data for an intended SAD of 1 μg/kg/min (median, 1.04, IQR 0.94–1.09). This led to median PAC of 0.30 μg/ml (IQR 0.14–0.63) among controls less than 240 min versus 0.77 μg/ml (IQR 0.74–0.97) in controls at least 240 min after argatroban initiation, respectively (p-value = 0.0749). Again, median SAD administered in patients with monitoring after at least 240 min (0.98 μg/kg/min) did not significantly differ from SAD applied in controls after less than 240 min (1.06 μg/kg/min, p-value >0.9999).
Magnitude and effect of argatroban dose adaptations on plasma concentration
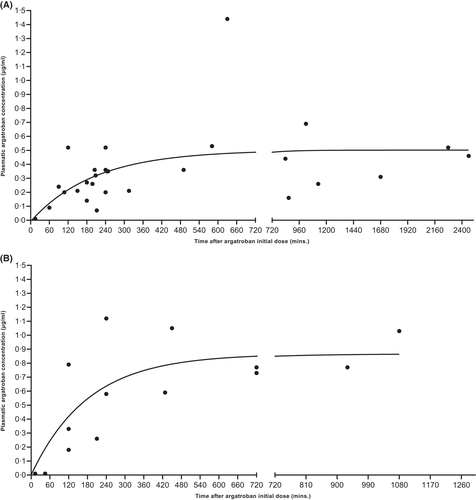
The most frequent dose adaptations in our cohort were ADI motivated by clinico-biological features, such as monitoring parameters below target ranges, insufficient improvement of platelet count and/or D-dimers, and thromboembolic events. Among patients with normal liver function, recorded ADI were divided into four intervals (+0.01–0.10, 0.11–0.20, 0.21–0.30, and 0.31–0.40 μg/kg/min). Figure 3A depicts PAC measured 3–6 h after ADI (median: 240 min, IQR: 217–297), showing that median PAC increases (μg/ml) corresponded roughly to the respective ADI (μg/kg/min).
Effect of timing of monitoring on plasma argatroban concentration
Among patients with normal liver function, ADI within a magnitude of 0.11–0.40 μg/kg/min were divided into two groups, based on the timing of monitoring: within 3 h (median, 128 min) or between 3 and 6 h (median, 240 min) after ADI. Figure 3B shows that late monitoring was associated with significantly higher PAC compared to early monitoring, despite the fact that, paradoxically, the median ADI performed in the group with PAC control within 3 h was significantly higher compared to the group with PAC control performed after 3–6 h (median, 0.22 vs. 0.21 μg/kg/min, IQR 0.21–0.26 vs. 0.14–0.22, p-value = 0.048).
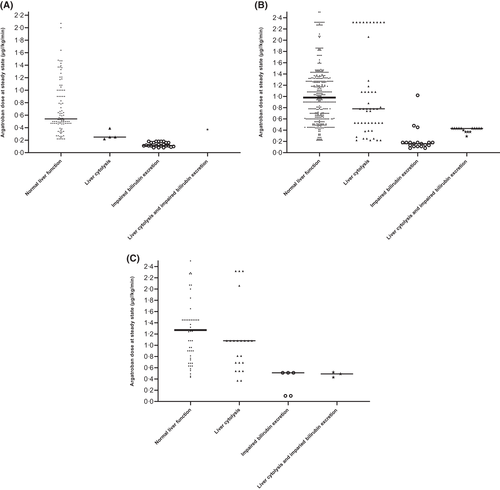
Argatroban dosing adapted to liver function
Steady-state PAC was defined as no argatroban dose modification in the 6 h preceding monitoring. Patients were divided into four categories according to their estimated liver function. For each group, we created PAC intervals according to the treatment aim: (i) ‘prophylactic’ PAC, 0–0.39 μg/ml (Figure 4A); (ii) ‘standard therapeutic’ PAC, 0.40–0.99 μg/ml (Figure 4B); and (iii) ‘high therapeutic’ PAC, 1.00–1.5 μg/ml (Figure 4C). At steady state, patients with normal liver function required median (IQR) argatroban doses of 0.54 μg/kg/min (0.47–1.0), 0.98 μg/kg/min (0.61–1.32), and 1.27 μg/kg/min (0.90–1.45) to reach prophylactic, standard therapeutic, or high therapeutic PAC, respectively. Patients with liver cytolysis required argatroban doses of 0.25 μg/kg/min (0.24–0.29), 0.78 μg/kg/min (0.53–1.10), and 1.08 μg/kg/min (0.69–1.08) respectively, and patients with hyperbilirubinaemia, argatroban doses of 0.12 μg/kg/min (0.11–0.16), 0.16 μg/kg/min (0.12–0.18), and 0.51 μg/kg/min (0.10–0.51).
Median argatroban doses were significantly lower in patients with hyperbilirubinaemia compared to patients with normal liver function or liver cytolysis and this was irrespective of the PAC interval. Moreover, among patients with liver cytolysis, median argatroban doses were significantly lower compared to those in patients with normal liver function in the ‘prophylactic’ and ‘high therapeutic’ PAC intervals.
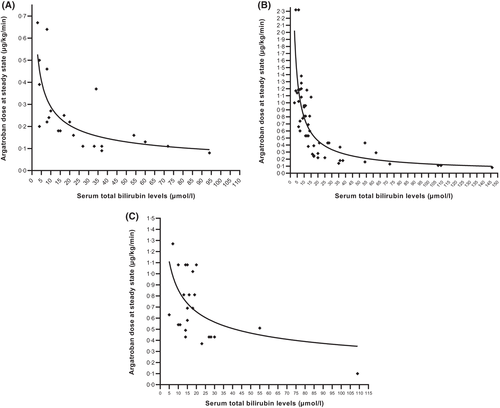
Total bilirubin levels and argatroban doses
Figure 5 depicts argatroban doses at steady state and concomitant bilirubin level, according to the targeted PAC. Among patients that had ‘prophylactic’ (0–0.39 μg/ml; Figure 5A) and ‘standard therapeutic’ (0.40–0.99 μg/ml; Figure 5B) PAC, serum total bilirubin levels and argatroban doses at steady state were strongly inversely correlated [Spearmann r (95% confidence interval (CI)): −0.8203 (−0.9156; −0.6376), p-value: <0.0001, n = 28 pairs and −0.8728 (−0.9185; −0.8041), p-value: <0.0001, n = 77 pairs, respectively]. Inverse correlation was lower among patients that had a ‘high therapeutic’ (≥1.0 μg/ml; Figure 5C) PAC [Spearmann r (95% CI): −0.5334 (−0.7644; −0.1809), approximate p-value: 0.0042, n = 27 pairs].
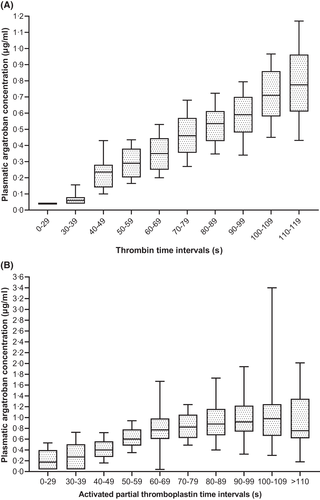
Monitoring of argatroban plasma concentration with routine assays
Three different assays for argatroban anticoagulation monitoring were available: aPTT (s), TT (s), and PAC (μg/ml). PAC and corresponding simultaneous TT and aPTT were analysed. PAC and corresponding single TT values showed the highest Spearman correlation [ρ (95% CI): 0.726 (0.689–0.7599); n = 749 pairs], while aPTT and simultaneous PAC showed a lower correlation [ρ (95% CI): 0.590 (0.548–0.630); n = 1031 pairs]. As shown in Figure 6A, increasing TT intervals showed a linear trend, with 80% of TT values higher than 79 s reaching at least a therapeutic PAC (>0.4 μg/ml). Of note, while aPTT intervals within 30–69 s showed a linear trend with the corresponding PAC, aPTT intervals higher than 70 s plateaued (Figure 6B).
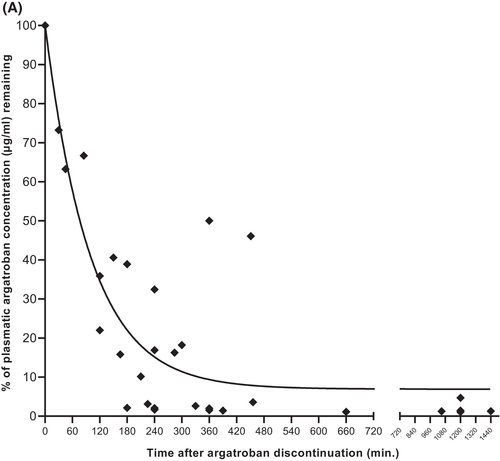
Plasma argatroban elimination
PAC was monitored after argatroban discontinuation in case of planned surgery, bleeding events, and switching to vitamin K antagonists (VKA). Residual PAC at time t′ after argatroban discontinuation were calculated as percentage of the last steady-state concentration and represented with a one-phase decay curve. We estimate that argatroban had an approximate plasma half-life of 60 min among patients with normal hepatic function (Figure 7A). Because of data scarcity, we could not analyse PAC decay after argatroban discontinuation among patients with hyperbilirubinaemia.
Impact of argatroban on values of the international normalised ratio
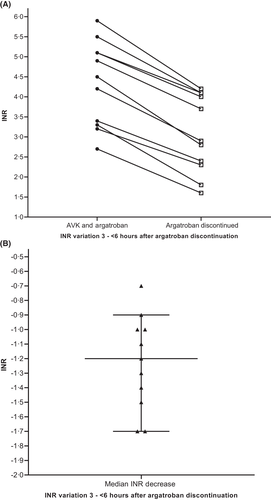
Figure 8 depicts median international normalized ratio (INR) among patients (n = 11) treated with VKA and argatroban. Median INR was 4.5 immediately before argatroban discontinuation and 2.9 measured 3– 6 h (median, 263 min) following argatroban discontinuation (median INR decrease −1.2, IQR INR decrease −0.7 to −1.7).
DISCUSSION
In order to develop evidence-based in-house recommendations for argatroban dosing and monitoring we analysed a cohort of 32 patients with confirmed HIT, about half of them (n = 15, 47%) presenting with HIT-T (Table 1). Median anticoagulation duration was 17.5 days (IQR 10–29.5, range 3–109) for a total of 729 treatment days. As depicted in Figure 1, argatroban was biologically effective, enabling progressive decrease of D-dimers and platelet count normalisation within 7–8 days of treatment, without causing a drop of haemoglobin levels. These results are in line with those presented in our previous publication.6
The effect of timing of first monitoring on the plasma argatroban concentration
Our results do not support the recommendation of performing the first laboratory control 2 h after starting argatroban.10, 15, 19 Among patients with normal liver function, a steady-state plasmatic concentration is reached at the earliest 4 h after starting argatroban, both in case of a SAD of 0.5 μg/kg/min (Figure 2A) and 1.0 μg/kg/min (Figure 2B). Based on our observations, we estimate a plasmatic argatroban half-life of approximately 1 h (Figure 7A), which agrees with published data.12 Our results are in line with those of Tardy-Poncet et al. who noted that argatroban concentrations measured 2 h after SAD were lower than steady-state concentrations determined later (i.e. 0.39 ± 0.29 μg/ml vs. 0.61 ± 0.28 μg/ml respectively).15 Based on these results and since four to five drug half-lives are necessary to reach plasma steady state after starting administration,20 we suggest performing the first PAC control at least 4 h after SAD.
The effect of the dose adjustments and timing of monitoring on subsequent plasma argatroban concentrations
To our knowledge, there is only one publication that assessed the magnitude of argatroban dose adaptations and their impact on consecutive PAC.6 Recent guidelines on management of patients with HIT did not give recommendations on this issue.10 Our data show that: (i) ADI intervals of 0.01–0.10, 0.11–0.20, 0.21–0.30, and 0.31–0.4 μg/kg/min lead to significant PAC increases (Figure 3A); (ii) a given ADI induced a roughly similar median PAC increase (i.e., Δ ADI μg/kg/min ≈ Δ PAC μg/ml); and (iii) significant differences in PAC increases were observed between ADI of 0.01–0.10 μg/kg/min and 0.21–0.30 μg/kg/min and between ADI of 0.01–0.10 μg/kg/min and 0.31–0.40 μg/kg/min.
Correct timing of laboratory monitoring is crucial for obtaining a reliable result. However, to the best of our knowledge, there are no published data concerning this practical issue.10 Similarly to what we have observed with initial monitoring (Figure 2), we show in Figure 3B that after ADI of 0.1–0.4 μg/kg/min, median PAC increase was significantly greater when laboratory control was performed ‘late’ (after 3 to up to 6 h) compared to ‘early’ controls (within 3 h). Hence, this underscores that timing of control after argatroban dose adaptation is critical15 and we suggest waiting 4 h after dose adaptation among patients with normal liver function.
The effect of liver function on argatroban dosing
The argatroban dose required to reach a given target PAC depends on liver function. As suggested by Levine et al.,13 serum total bilirubin might be more useful than ALT to identify patients who require dose reduction. Based on the alteration of bilirubin and/or ALT, we defined four different hepatic impairment patterns (Figure 4). Patients with increased bilirubin levels required significantly lower argatroban steady-state doses than patients with normal liver function irrespective of the target PAC interval. Patients with liver cytolysis tended to require lower median argatroban doses at steady state than patients with normal liver function, in order to reach prophylactic and high therapeutic PAC intervals. Our data confirm that impaired bilirubin excretion plays the most important role in decision-making for argatroban dosing and indicate that patients with liver cytolysis probably require smaller argatroban dose reductions compared to patients with impaired bilirubin excretion.
The extent of bilirubinaemia and argatroban dosing
A total plasma bilirubin level higher than 25.5 μmol/l has been proposed to identify patients with impaired bilirubin excretion, thus requiring argatroban dose reduction.13 However, as presented in Figure 5, we could not identify a critical serum total bilirubin threshold, observing a progressive impact on the required dosage in order to achieve a prophylactic (Figure 5A), standard therapeutic (Figure 5B), or high therapeutic PAC (Figure 5C) already at bilirubin values within reference range. Based on our data, we suggest to consider reducing argatroban doses among patients with serum total bilirubin levels above 20 μmol/l, which is lower than the threshold suggested by Levine et al.13
Argatroban laboratory monitoring with aPTT and thrombin time
Activated partial thromboplastin time is the most widely available laboratory assay for monitoring argatroban anticoagulation and is currently recommended by the American Society of Hematology (ASH) guidelines.10 However, multiple factors might affect its results, leading to inaccurate monitoring, which in turn may result in argatroban under-dosing and thrombosis progression.21 Our group and others have shown that TT is more reliable and robust for the monitoring of argatroban.6, 22 Figure 6A confirms a linear relationship between PAC and corresponding TT intervals. In contrast, the relationship between increasing PAC and correspondent simultaneous aPTT intervals is not linear, reaching a plateau for aPTT values above 70 s (Figure 6B), underscoring that this coagulation assay is less accurate for monitoring argatroban anticoagulation.6, 21, 23 We therefore suggest to monitor argatroban by TT when the assessment of PAC with a commercially diluted TT assay is not available. From an analytical point of view, it would be better to employ two TTs, e.g. with final thrombin concentrations of 1.5 and 5 U/ml as we did in our previous work.6, 24 However, our current experience indicates that also a single TT with a final thrombin concentration of 1.25 U/ml is adequate up to argatroban concentrations of about 1 μg/ml (Figure 6A). Monitoring argatroban with thrombin-time-based assays is in line with current expert's opinions.21
Argatroban half-life after discontinuation
We found an argatroban plasma half-life of about 1 h in patients with normal liver function (Figure 2A,B). Accordingly, Figure 7 confirms that the decay of PAC after argatroban discontinuation follows the same kinetics. We therefore suggest discontinuing argatroban at least 2 h before any invasive intervention among patients with normal liver function.
The effect of argatroban on the INR
As well as direct oral anticoagulants,9 VKA are still employed for HIT patients after the acute phase,5 targeting INR values between 2 and 3 for optimal anticoagulation.5 Of note, argatroban and other direct thrombin inhibitors increase INR values; this implies that during the overlap phase of anticoagulation with both argatroban and VKA, INR values greater than 2–3 have to be achieved before stopping argatroban.25 Harder et al.26 recommend to target INR values higher than 4 and Bartholomew et al.27 recommend performing INR controls 6 h after argatroban discontinuation. In Figure 8, we show that INR values ranging from 3.5 to 4.5 during the overlapping phase are sufficient to obtain a target INR of 2–3 after argatroban discontinuation. Indeed, we observed a median INR decrease of 1.2 (range: −0.7; −1.7) when control was performed 3–6 h after argatroban discontinuation. These data are perfectly in line with those obtained by two other groups who observed median INR decreases of 1.2.6, 28
Limitations of our study
Our study has limitations. Firstly, this was a single-centre study. Secondly, our data were collected retrospectively. Thirdly, argatroban monitoring with a commercial diluted TT is not widely available. Nevertheless, all HIT cases were confirmed by a functional gold standard assay, we studied patients treated in a real-world clinical setting and analysed an argatroban treatment length of 729 days. Moreover, to the best of our knowledge, our cohort is the biggest ever described including patients with HIT confirmed by a functional gold standard assay who were treated with argatroban.6, 11, 29-32
Practical implications of our study
Our results provide guidance on some practical aspects of anticoagulation of HIT patients with argatroban, such as: (i) the effect of various argatroban starting doses and dose adjustments on the following plasma concentrations; (ii) the effect of liver function on argatroban dosing; (iii) the effect of timing and laboratory assay on monitoring; and (iv) the effect of therapeutic argatroban on INR values. Based on these data we have developed a standardised in-house management protocol (Table 5), which we are prospectively validating. Briefly, we do not differentiate between HIT and HIT-T and employ a SAD of 1.0 μg/kg/min in all HIT patients with normal liver function. In patients with impaired liver function, we decrease SAD to 0.75 μg/kg/min in presence of isolated hepatic cytolysis and to 0.25 μg/kg/min in case of hyperbilirubinaemia. We monitor treatment with a double pharmacokinetic and pharmacodynamic approach. On one side, we assess PAC at steady state employing a thrombin-time-based assay, which is more robust than aPTT. On the other side, we follow the course of platelet counts and D-dimer levels in order to fine-tune the anticoagulation intensity required in each individual patient.
| Normal liver function | Impaired liver function | Multiorgan dysfunctiona | ||
|---|---|---|---|---|
| Impaired bilirubin excretion | Isolated liver cytolysis | |||
| Monitoring of platelets and D-dimers | Once daily | Once daily | Once daily | Once daily |
| Monitoring of hepatic function | Before SAD, then at discretion of physician | Before SAD, then at discretion of physician | Before SAD, then at discretion of physician | Before SAD, then at discretion of physician |
| SAD (μg/kg/min) | 1.0 | 0.2513 versus contra-indicatedb | 0.75c | 0.50 |
| Timing of monitoring after SAD (hours) | 4 h; repeat twice at 4 h each if no ADA, afterwards once daily in ss | 6 h; repeat twice at 6 h each if no ADA, afterwards once daily in ss | 4 h; repeat twice at 4 h each if no ADA, afterwards once daily in ss | 4 h; repeat twice at 4 h each if no ADA, afterwards once daily in ss |
| ss-PAC target (μg/ml) | 0.5–1.0d | 0.5–1.0d | 0.5–1.0d | 0.5–1.0d |
| ss-TT target (s) | 80–120 | 80–120 | 80–120 | 80–120 |
| ss-aPTT target (fold increase and s) |
1.7–2.4 × baseline value (max. 70–75 s)e |
1.7–2.4 × baseline value (max. 70–75 s)e |
1.7–2.4 × baseline value (max. 70–75 s)e |
1.7–2.4 × baseline value (max. 70–75 s)e |
| Magnitude of ADA (μg/kg/min) | Adapted to first PAC/TT and to ss-PAC-targeted; Δ ADA μg/kg/min ≈ Δ PAC μg/ml (at least 0.2 μg/kg/min) | Adapted to first PAC/TT and to ss-PAC -targeted; Δ ADA μg/kg/min ≈ 2Δ PAC μg/ml (0.05–0.1 μg/kg/min) | Adapted to first PAC/TT and to ss-PAC - targeted; Δ ADA μg/kg/min ≈ Δ PAC μg/ml (0.2 μg/kg/min) | Adapted to first PAC/TT and to ss-PAC-targeted; Δ ADA μg/kg/min ≈ Δ PAC μg/ml (0.2 μg/kg/min) |
| Timing of monitoring after ADA | 4 h; repeat 4 h after each ADA | 6 h; repeat 6 h after each ADA | 4 h; repeat 4 h after each ADA | 4 h; repeat 4 h after each ADA |
| Frequency of PAC/TT/aPTT monitoring | Once daily after 3 target ss-PAC/TT | Once daily after 3 target ss-PAC/TT | Once daily after 3 target ss-PAC/TT | Once daily after 3 target ss-PAC/TT |
| When bridging to AVK: target INR on argatroban | 3.5–4.5 | 3.5–4.5 | 3.5–4.5 | – |
| When bridging to AVK: timing of INR after argatroban stop | After 4 h | After more than 4 h | After 4 h | – |
- Abbreviations: ADA, argatroban dose adaptation; aPTT, activated partial thromboplastin time; AVK, vitamin K antagonist; INR, international normalised ratio; SAD, starting argatroban dose; ss, steady state; TT, thrombin time (see Methods).
- a Multi-organ dysfunction without impaired bilirubin excretion.
- b Use SAD of 0.25 μg/kg/min among patients with mild to moderate bilirubin excretion impairment and consider danaparoid for patients with severe bilirubin excretion impairment.
- c See results in paragraph "Argatroban dosing adapted to liver function".
- d Target higher PAC (1–1.5 μg/ml) in case of insufficient platelet count recovery and/or D-dimers level decrease.
- e Reagent- and coagulometer-specific values, not generalisable.
ACKNOWLEDGEMENTS
We thank the physicians caring for the patients, the technicians of the haemostasis laboratory, Prof. MD Michel Duchosal for support, and Prof. MD Pierre-Alexandre Bart for acting as expert of the MD thesis of Matteo Marchetti. This study has been partially presented at the Swiss Oncology and Haematology Congress (SOHC) in Zürich, Switzerland, June 2019. Open Access Funding provided by Universite de Lausanne. [Correction added on 26 May 2022, after first online publication: CSAL funding statement has been added.]
CONFLICT OF INTEREST
The authors do not have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Matteo Marchetti designed the research, collected and analysed data, and wrote the manuscript. Stefano Barelli designed the research and cowrote the manuscript. Tobias Gleich and Francisco J. Gomez collected data. Matthew Goodyer cowrote the manuscript. Francesco Grandoni was in charge of the patients, analysed data and cowrote the manuscript. Lorenzo Alberio is the corresponding author of the manuscript. He was in charge of the patients, designed the research, analysed data, and wrote the manuscript. All authors read and approved the final version of the manuscript.