Diagnosis and management of Waldenström macroglobulinaemia—A British Society for Haematology guideline
Abstract
Scope
The objective of this guideline is to provide healthcare professionals with clear guidance on the management of patients with Waldenström macroglobulinaemia. In individual patients, circumstances may dictate an alternative approach.
Methodology
This guideline was compiled according to the British Society for Haematology (BSH) process at http://www.b-s-h.org.uk/guidelines/proposing-and-writing-a-new-bsh-guideline/. Recommendations are based on a review of the literature using Medline, Pubmed, Embase, Central, Web of Science searches from beginning of 2013 (since the publication of the previous guidelines) up to November 2021. The following search terms were used: Waldenström(’s) macroglobulin(a)emia OR lymphoplasmacytic lymphoma, IgM(-related) neuropathy OR cold h(a)emagglutinin disease OR cold agglutinin disease OR cryoglobulin(a)emia AND (for group a only) cytogenetic OR molecular OR mutation OR MYD88 OR CXCR4, management OR treatment OR transfusion OR supportive care OR plasma exchange OR plasmapheresis OR chemotherapy OR bendamustine OR bortezomib OR ibrutinib OR fludarabine OR dexamethasone OR cyclophosphamide OR rituximab OR everolimus, bone marrow transplantation OR stem cell transplantation. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) nomenclature was used to evaluate levels of evidence and to assess the strength of recommendations. The GRADE criteria can be found at http://www.gradeworkinggroup.org. Review of the manuscript was performed by the British Society for Haematology (BSH) Guidelines Committee Haemato-Oncology Task Force, the BSH Guidelines Committee and the Haemato-Oncology sounding board of BSH. It was also on the members section of the BSH website for comment. It has also been reviewed by UK Charity WMUK; these organisations do not necessarily approve or endorse the contents.
INTRODUCTION
Waldenström macroglobulinaemia (WM) is a distinct B-cell lymphoproliferative disorder characterised by an immunoglobulin IgM monoclonal gammopathy and bone marrow infiltration by lymphoplasmacytic lymphoma (LPL).1, 2 Clinical features may be related to overall disease burden, such as anaemia, constitutional symptoms, or may be directly attributable to the IgM paraprotein. The term IgM-related disorders denotes the presence of clinical features attributable to the IgM paraprotein in the absence of bone marrow lymphoplasmacytic infiltration.1
A precursor condition, IgM monoclonal gammopathy of undetermined significance (MGUS), is defined by having all of the following criteria: (1) the presence of an IgM paraprotein of less than 30 g/L; (2) absence of a lymphoplasmacytic bone marrow infiltration; and (3) absence of signs or symptoms such as occur in WM itself.1 The rate of transformation for an individual with IgM MGUS to WM is approximately 1%–2% per year.3 IgM paraproteins can be seen with other B lymphoproliferative disorders such as marginal zone lymphoma or chronic lymphocytic leukaemia (CLL).
WM is more common in the elderly and Caucasians and has a male predominance. There is an increased risk of WM when there is a personal or family history of a wide range of autoimmune (Sjögren syndrome, autoimmune haemolytic anaemia), inflammatory and infective disorders or other B-cell disorders amongst relatives of patients with WM but screening of family members is not recommended due to low absolute risk.4, 5
DIAGNOSIS AND INVESTIGATIONS
Baseline laboratory investigations
A list of useful investigations for patients with suspected or established WM is provided in Table 1. Further guidance is provided by the international taskforce recommendations.6 Anaemia due to marrow infiltration is common at presentation but other causes of anaemia need excluding. Tests for neuropathy, cryoglobulinaemia, amyloidosis, cold agglutinins, bleeding diathesis and central nervous system (CNS) disease should be tailored to the clinical scenario (Table 1).
| Clinical indication | Suspected complication | Suggested investigations |
|---|---|---|
| At diagnosis |
FBC Urea and creatinine Liver function tests LDH β2 microglobulin Hepatitis B, C, HIV status Serum protein electrophoresis and immunofixation Quantification of IgM by densitometry Quantification of IgG and IgA SFLC Plasma viscosity Ophthalmic examination for signs of hyperviscosity Bone marrow aspirate and trephine biopsy
|
|
| Prior to treatment |
Baseline CT neck chest abdomen and pelvis if symptomatic Repeat paraprotein quantification Virology (hepatitis B, C, HIV) Consider bone marrow aspirate and trephine biopsy |
|
| Anaemia | Haematinics, haemolysis screen (reticulocyte count, LDH, haptoglobin, bilirubin), DAT | |
| Bleeding | Hyperviscosity |
Paraprotein quantification Plasma viscosity |
|
Acquired VWD Amyloidosis (acquired factor deficiency) |
Coagulation studies VWD screen Factor assays SFLC |
|
| Lymphocytosis | Flow cytometry to confirm if PB involvement | |
| Neuropathy |
Peripheral neuropathy Cryoglobulinaemia Amyloidosis POEMS |
Anti-MAG titre Anti-ganglioside antibody Additional anti-neuronal antibodies in discussion with peripheral nerve specialist Cryoglobulin SFLC VEGF Nerve conduction studies Lumbar puncture for CSF analysis
Nerve biopsy |
| Skin rash/purpura/Raynaud phenomenon/ulceration |
CAD Cryoglobulinaemia Schnitzler's syndrome |
DAT Haemolysis screen Cryoglobulins Skin biopsy |
| Renal impairment |
Cryoglobulinaemia Amyloidosis |
Cryoglobulins 24-h urine protein SFLC Renal biopsy |
| Suspected amyloidosis |
SFLC Biopsy of involved tissue Congo red stain for amyloid SAP scan Assessment of organ function
|
|
| Suspected high grade transformation |
PET-CT LDH Biopsy of suspected area of transformation |
|
| Suspected Bing–Neel syndrome |
Brain and whole-spine MRI with gadolinium contrast (if renal impairment consult cardiologist) Lumbar puncture for CSF
|
- Abbreviations: BNP, brain natriuretic peptide; CAD, cold agglutinin disease; CSF, cerebrospinal fluid; CT, computed tomography; DAT, direct antiglobulin test; FBC, full blood count; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; PB, peripheral blood; PET, positron emission tomography; SFLC, serum free light chains; VWD, von Willebrand disease, IgH Immunoglobulin gene, PCR, polymerase chain reaction; SAP, serum amyloid P; VEGF, vascular endothelial growth factor.
IgM paraproteins should be demonstrated by serum protein electrophoresis and quantitated by densitometry. Assessment of total IgM concentration by nephelometry is a viable alternative to densitometric assessment of paraprotein concentration although the former provides systematically higher values.7, 8 Concentrations of IgG and IgA should also be determined at diagnosis and at regular intervals during follow-up.9 It is optimal that the sequential assessment of paraprotein concentration be performed by the same method within the same laboratory.10
The serum free light chain (SFLC) assay has been evaluated by a number of investigators and approximately 80% of patients have elevated levels of involved free light chain (median values 48.6–103.5 mg/L) but its use in monitoring is limited clinically to those rare situations where the light chain is causing cast nephropathy, renal gammopathy or amyloid light-chain (AL) amyloidosis, or the IgM component is difficult to measure (e.g., cryoglobulin).11-13 Rarely an artefactually elevated SFLC occurs due to polymerisation of the light chain although it still provides monitoring information for that patient.
Recommendations
- Sequential monitoring of IgM/ monoclonal protein should be performed in a single laboratory using a single methodology (Grade A1).
- There is no evidence currently to support the use of SFLC assessment for routine monitoring outside clinical trials (Grade C2).
- Tests for neuropathy, cryoglobulinaemia, amyloidosis, cold agglutinins, bleeding diathesis and CNS disease should be tailored to the clinical scenario (Table 1) (Grade A1).
- Alternative causes apart from WM should also be considered for symptoms and should be investigated where appropriate (Grade A1).
- Screening for hepatitis B and C and human immunodeficiency virus (HIV) is required prior to the introduction of treatment (Grade A1).
Pathological diagnosis and genomic assessment
Bone marrow assessment is central to the pathological diagnosis of WM.1 It is recommended in all patients suspected of having symptomatic WM or another IgM-related disorder. The value of assessment in asymptomatic individuals is not fully established but may provide prognostic information with respect to risk of progression and this can be discussed with individual patients.14, 15 A bone marrow aspirate and trephine biopsy are recommended for the majority of patients. The trephine should be reported by a specialist haematopathologist in line with NICE guidance (NG47). A trephine biopsy provides better overall assessment of disease burden and also allows clearer demonstration of plasmacytic differentiation as well as other diagnostic clues such as the presence of reactive mast cells and immunoglobulin inclusions.
WM is characterised by the presence of monotypic B cells and plasma cells and the IgM concentration appears to correlate with the extent of plasma-cell differentiation rather than overall bone marrow disease burden.16, 17 B cells have a CD22wk CD25+ immunophenotype which can only be assessed by flow cytometry. In addition to providing diagnostic value in symptomatic patients, bone marrow assessment is a useful adjunct in the assessment of patients with IgM-related disorders that are typically characterised by a low disease burden and it may also provide prognostic information in asymptomatic patients.18
In 2012, Treon and colleagues demonstrated by whole-genome sequencing that >90% of WM patients harboured a single point mutation in MYD88, the L265P mutation.19 This appears central to the pathogenesis of WM and leads to the downstream activation of NF-κB via divergent pathways including Bruton tyrosine kinase (BTK) and IRAK1/IRAK4.19, 20
Although not specific to WM, the demonstration of MYD88L265P has clear diagnostic utility allowing a precise genomic diagnosis in the correct clinical and pathological context. There is no clear consensus on methodologies although allele-specific polymerase chain reaction (PCR), digital droplet PCR and high-throughput sequencing methodologies have all been described.21, 22 Laboratories are encouraged to establish the level of sensitivity of their assays and this should be reported so that results can be correlated with the level of bone marrow as assessed by morphology and flow cytometry. This is particularly relevant for MYD88WT (wild-type) disease which appears to be characterised by a greater risk of histological transformation and shorter survival.23 MYD88 status may also influence the outcome with BTK inhibitors.24
Mutation of CXCR4 is seen in 30%–40% of WM patients.25 A range of CXCR4 mutations have been identified, these are subclonal with highly variable allele frequencies and some patients acquire multiple mutations. This can present a significant challenge in routine diagnostic laboratories and so high-throughput technologies following B-cell selection are likely to provide the optimal approach.25, 26 It has been suggested that CXCR4 genotype influences clinical features and response rates, kinetics of response and progression-free survival (PFS) in ibrutinib-treated patients.27, 28 This effect however is complex and is influenced by mutation type (frameshift versus nonsense) and allele burden and may be, at least in part, overcome by the addition of rituximab.29-31 The significance of CXCR4 mutation has not been established in patients treated with conventional therapies, with only one group reporting no difference in outcome with chemoimmunotherapy albeit in small cohorts of patients.32 Similarly, they do not appear to influence outcome with proteasome inhibitor-based therapy.33, 34
TP53 abnormalities (17p deletion and/or gene mutation) are noted in a significant minority (up to 11%) of WM patients at diagnosis and are associated with significantly inferior progression-free and overall survival.35, 36 They also appear to predict shorter times to progression in patients with asymptomatic disease. TP53 mutations may also be acquired during the course of the disease.35, 37 Assessment of TP53 for both 17p deletion and gene mutation could potentially influence treatment decisions. As above, B-cell selection and high-throughput sequencing approaches are considered optimal for detecting TP53 mutation or loss.
Recommendations
- Bone marrow aspirate and trephine biopsy is needed to make a definitive diagnosis of WM and is recommended in all patients with suspected symptomatic WM or other IgM-disorder. (Grade A1)
- Flow cytometry is preferred method for establishing B-cell immunophenotype. (Grade A1)
- In all patients undergoing bone marrow assessment, MYD88 L265P should be assessed using an assay of established sensitivity. (Grade A1)
- Assessment of CXCR4 and TP53 should be considered and should be performed prospectively in all clinical trials. (Grade A1)
Imaging
Lymphadenopathy and splenomegaly are relatively infrequent in patients with WM, being reported in approximately 15% of patients prior to starting treatment but are more common with later progressions.38 Extramedullary WM is rare and has been described with a frequency of <5% but this occurs mainly at relapse following previous treatment and high-grade transformation needs exclusion in this setting.39 Baseline computed tomography (CT) imaging is regarded as standard practice in symptomatic patients prior to commencing therapy. There are limited data on the utility of fluorodeoxyglucose–positron emission tomography (FDG-PET-CT) scanning in WM. Banwait et al. demonstrated that PET-CT is informative in 80% of patients which may be higher than CT assessment but there is no convincing rationale for routine use of PET-CT and further prospective study is required.40 FDG-PET imaging may have a role in the assessment of patients with suspected histological transformation.
Recommendations
- CT scan (neck, chest, abdomen, pelvis) is recommended in all patients prior to the commencement of each line of therapy (Grade B1).
- The value of FDG-PET remains to be determined and is not recommended outside of a clinical trial. It may have a role in detection of high-grade transformation (Grade C2).
Prognostic assessment
The international prognostic scoring system for WM (ISSWM) is based on the assessment of five adverse prognostic features, namely age >65 years, haemoglobin concentration ≤115 g/L, platelets ≤100 × 109/L, β2 microglobulin (B2M) >3 mg/L and paraprotein concentration >70 g/L at the time of initiation of treatment. This system defines three risk groups with 5-year overall survival (OS) of 87%, 68% and 36%.41 However this evaluation predated the use of novel agents such as ibrutinib and proteasome inhibitors. There is an international consensus that the ISSWM should be recorded in clinical trials but there is no evidence that it should influence treatment decisions for individual patients.
More recently a revised prognostic scoring system based on age (≤65 vs. 66–75 vs. ≥76 years), B2M ≥ 4 mg/L, serum albumin <35 g/L, and lactate dehydrogenase (LDH) ≥250 iu/L (upper limit of normal [ULN] <225) has also been proposed, but this will require further validation.42
The impact of genomic profile on OS is still unknown, especially given the advent of targeted therapies. There is conflicting evidence from retrospective data sets as to whether patients with MYD88WT have a shorter OS than those with MYD88L295P.23, 27, 43 As described above, TP53 mutation/TP53 loss, although rare, is associated with an inferior survival.35, 37
Recommendation
- Whilst potentially useful to guide discussions with patients, there is no evidence to support the use of ISSWM or other prognostic scoring systems in determining treatment approaches for individual patients and they need further validation in the era of newer agents (Grade B1).
TREATMENT
Indications for treatment
A significant proportion of WM patients are asymptomatic at presentation and can be safely observed at 3–6-monthly intervals. The risk of progression to symptomatic disease is 59% at 5 years.44 A progression risk stratification for patients with asymptomatc WM has been created to predict time to treatment (see link www.awmrisk.com).45
The indications for the introduction of treatment include constitutional symptoms, symptomatic or bulky lymphadenopathy or splenomegaly, cytopenias secondary to marrow infiltration, paraprotein-related indications including hyperviscosity, and IgM-related syndromes such cryoglobulinaemia, amyloidosis, peripheral neuropathy and cold agglutinin disease (CAD).46
Treatment
Developing evidence-based treatment algorithms in WM is hindered by a lack of robust data. The majority of published studies are non-randomised, often single-institution-based phase II studies that include both de novo and relapsed disease, and more general studies of B-cell non-Hodgkin lymphoma (B-NHL) rather than specifically WM. Optimal choice and sequence of therapies is therefore unknown. Patients should be considered for clinical trials where possible.
Chemoimmunotherapy
Rituximab monotherapy is generally well tolerated but associated with modest response rates and relatively short PFS but may be considered in frail patients considered unsuitable for combination therapies if BTK inhibitors (BTKi) are unavailable.47 Rituximab can also be associated with a paradoxical rise in IgM, the so-called IgM flare phenomenon.48, 49 The risk of flare is lower with combination therapies but pre-emptive plasma exchange or deferring rituximab is recommended in those patients with high baseline IgM.50
Rituximab combination therapies are the cornerstone of first-line treatment in WM with response rates typically over 80%. The two most commonly used first-line regimens are dexamethasone, rituximab and cyclophosphamide (DRC) and rituximab–bendamustine (BR).
DRC is the alkylator–rituximab combination for which there are trial data with the longest follow-up, with excellent efficacy and a favourable toxicity profile.51, 52 With a median follow-up of 8 years, the final analysis of a multicentre study of 72 patients for front-line therapy showed an 83% response rate, with a median time to response, PFS and time to next treatment (TTNT) of 4, 35 and 51 months respectively. Eight-year OS was 32%, but importantly 43% of deaths were unrelated to WM. Toxicity was remarkably low, with only 9% grade 3/4 neutropenia, minimal thrombocytopenia and only 14% grade ≥3 infectious episodes. Similar efficacy outcomes have been reported in the “real world” setting.53
The combination of BR for front-line therapy has been studied as part of a randomised trial comparing rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R-CHOP) and BR in patients with indolent lymphomas.54 The WM subgroup of this study consisted of 43 patients of whom 22 had BR, with this group having a significantly longer PFS (69.5 vs. 28.1 months) and marked less toxicity. A similar PFS of 65 months was seen in the follow-on study, StiL NHL7-2008 MAINTAIN trial, in the 179 patients who had BR alone.55 A French retrospective multicentre study in 69 patients demonstrated a very high response rate of 97% with a 2-year PFS of 87%.32 Approximately half of the patients completed the six cycles of standard dose BR (bendamustine 90 mg/m2) but the remainder required dose reductions or fewer cycles. Reassuringly there were only 10 grade 3/4 haematological toxicity events and eight grade 3/4 infectious episodes. Importantly the toxicities that led to a reduction of BR dose intensity in 44% of patients did not impair survival. Overall, when considering BR as an induction regimen, a dose reduction and/or reduction of the number of cycles could be considered in the frail and elderly but may affect duration of response.56 There is also retrospective evidence of this combination's efficacy in the relapsed and refractory setting with response rates of 80%–83% and a median PFS of 13 months and not reached, although both studies have relatively short follow-up.57, 58
There are no prospective comparative data of DRC versus BR but there are retrospective data that suggest that BR may offer a longer PFS with the trade-off of greater toxicity (in some circumstances).59 In this single-centre retrospective study, the outcomes of 160 consecutive patients, of whom 93 had relapsed or refractory WM and who were treated with either BR (n = 60) or DRC (n = 100), were analysed. In the treatment-naïve setting, median time to best response was 6.1 months in the BR group versus 11 months with DRC (p = 0.13). There was no difference in overall response rate (ORR, defined as minor response or better) between the two groups (93% vs. 96%). With a median follow-up of 30 months, the 2-year PFS was 88% and 61% for BR and DRC (p = 0.07) respectively. In the relapsed and refractory setting, the median time to best response was 7 months for both treatment regimens. The ORR was 95% and 87% with BR and DRC respectively (p = 0.45), and median PFS was 58 vs. 32 months, and 2-year PFS was 66% vs. 53% (p = 0.08).
Purine analogue combinations (e.g., fludarabine and rituximab +/− cyclophosphamide) are noted to have significant efficacy with a median PFS exceeding 50 months.60-63 However, the use of these combinations as first-line treatment is not recommended because of the risk of long-lasting cytopenias and secondary malignancies, particularly myelodysplastic syndromes.64
Single-agent chlorambucil has a very limited role in contemporary first-line therapy, however, it can provide a well-tolerated oral option in frail patients who are considered unsuitable for chemoimmunotherapy, rituximab monotherapy or BTKis or if they are unavailable.
Retrospective data suggested an improvement in PFS and OS with the use of maintenance rituximab; however, this was a non-randomised study.65 The role of maintenance rituximab was explored in a phase III randomised prospective study, StiL NHL7-2008 MAINTAIN, following front-line BR chemotherapy.55 Patients who achieved a partial response or better were randomised between rituximab maintenance and observation; with a median follow-up of 70 months, there was no statistical difference in median PFS between those who had maintenance rituximab (n = 109, 101 months) and those who were randomised to observation alone (n = 109, 83 months) with a hazard ratio (HR) of 0.80 (95% confidence interval [CI] 0.51–1.25) and no difference in OS.
Proteasome inhibitors
Proteasome inhibitors are highly active in WM and bortezomib, carfilzomib and ixazomib with rituximab combinations have been studied. Bortezomib, dexamethasone and rituximab (BDR) is the combination with the most robust data and longest follow-up. Bortezomib was given intravenously in this study, initially twice weekly followed by a weekly dosing schedule for a total treatment duration of 23 weeks.66 The ORR of 59 patients was 85% and after a median follow-up of 6 years, the median PFS was 43 months and OS at 7 years was 66%.67 The toxicity profile was extremely favourable except for 25% of patients who developed at least grade 2 neuropathy and 7% who developed grade 3 or above. In the National Cancer Research Institute (NCRI) randomised phase II study, R2W, 42 patients were randomised to BCR (subcutaneous weekly bortezomib with cyclophosphamide and rituximab), with an ORR of 98% and a 3-year PFS of 81%. There were no cases of grade 3 or higher neuropathy.64 The addition of bortezomib to the DRC backbone did not lead to a significant improvement in the 2-year PFS compared to DRC alone in a large randomised phase 2 study, 80.6% vs. 72.8% respectively.68
Bortezomib has been studied in the relapsed setting demonstrating good and rapid responses. Early studies evaluated bortezomib as a single agent in the conventional biweekly intravenous schedule and demonstrated ORR of 26%–96%, but neurotoxicity was common with 20%–30% experiencing grade 3 toxicity.69-71 Additionally, it was noted that some patients demonstrated a discrepancy between their IgM and lymph node/bone marrow responses. Subsequent studies incorporated rituximab and gave the bortezomib in a weekly schedule.72, 73 These schedules appear effective with a major response rate (partial response or better) of approximately 50% and a lower incidence of neurotoxicity (5% grade 3 or above) with a median PFS of 15.6 months noted in the larger of the two studies. Hence the use of the weekly schedule and subcutaneous route appears to reduce the risk of neurotoxicity. Recent data demonstrate CXCR4 genotype is not predictive of outcome to bortezomib.34
Newer proteasome inhibitors with different toxicity profiles and alternative routes of administration may become treatment options in the future. Carfilzomib, a second-generation proteasome inhibitor with limited neurotoxicity, has been investigated in combination with dexamethasone and rituximab (CaRD) in 31 patients with additional maintenance of rituximab and carfilzomib for 1 year with an ORR of 80% and a median PFS of 46 months.74, 75 Ixazomib, an oral proteasome inhibitor in combination with dexamethasone and rituximab (IDR) has shown promise in the front-line setting in a phase 2 trial (n = 26), with an ORR of 96% and a major response rate (MRR) of 77% and is a well-tolerated neuropathy-sparing regimen.76 The updated results of this trial after a median follow-up of 52 months has confirmed the durability of this regimen with a median PFS of 40 months.77
BTK inhibitors
The efficacy of BTK inhibitors has been demonstrated in multiple trials for patients with both treatment-naïve and previously treated WM. The pivotal phase 2 multicentre study evaluated the efficacy and tolerability of continuous ibrutinib 420 mg once daily in 63 patients with relapsed/ refractory (R/R) WM.24 The ORR in the whole cohort was 90% and with an extended follow-up of 59 months, 27 patients remain on treatment with a 5-year PFS of 54%.28 The same group conducted a single-centre study of 30 patients receiving ibrutinib for front-line therapy and reported a MRR of 87% and a 4-year PFS of 76%.78, 79 Atrial fibrillation (AF) occurred in 12%–20% in both studies, although the majority of patients were able to continue ibrutinib along with medical management of the AF and no new safety signals were identified in these small cohorts.
The combination of rituximab and ibrutinib has been evaluated in the iNNOVATE study where 150 patients with treatment-naïve (n = 68) or relapsed refractory WM requiring therapy (n = 82) were randomised to ibrutinib and rituximab versus rituximab plus placebo.29 The 4-year PFS rate in the treatment-naïve cohort was 70% and 32% respectively and in those previously treated, 71% and 20%.80 A subsidiary study of this trial investigated the role of ibrutinib alone in 31 patients with rituximab-refractory disease. After a median follow-up of 58 months, the median PFS was 39 months (95% CI 25—not evaluable).81
At the current time, it is not clear what additional benefit rituximab provides when added to ibrutinib in either the up-front or relapsed setting although it may abrogate the negative impact of CXCR4 mutations. Similarly, there are no prospective data comparing BTKi and more conventional rituximab–chemotherapy approaches in the up-front setting. In the UK this will be addressed in the recently initiated RAINBOW trial (ClinicalTrials.gov Identifier: NCT04061512) comparing DRC and rituximab + ibrutinib.
A retrospective comparison of 157 patients receiving ibrutinib off trial compared to 72 receiving it on trial found a non-inferior response in those treated outside a clinical trial with an estimated 4-year PFS in these patients of 63%.82 Similar to CLL, there is a suggestion from a retrospective analysis that maintaining dose intensity of ibrutinib is important to optimise outcomes.83 It has also been reported that approximately 20% of WM patients will experience ibrutinib withdrawal symptoms (fever, arthralgia, body aches and headache) which typically resolve on the re-introduction of ibrutinib.84 In the majority of patients who experience an IgM flare with ibrutinib interruption, IgM levels may not return to baseline for up to 3 months or longer.
A phase 1/2 study investigating zanubrutinib in 24 treatment-naïve patients and 53 patients with relapsed/refractory disease demonstrated an ORR of 96% and an estimated 3-year PFS of 80% with an acceptable toxicity profile: whilst 91% of patients had an infection, only a quarter were grade 3.85 Grade 3 or higher neutropenia was reported in 15% of patients. ASPEN, a large multicentre international randomised trial, compared zanubrutinib to ibrutinib in 201 patients with mutated MYD88 R/R WM or treatment-naïve if unsuitable for chemoimmunotherapy.86 The primary end-point of statistical superiority related to deep response (complete response/very good partial response [CR/VGPR]) was not met (28.4% vs. 19.2% for those receiving zanubrutinib and ibrutinib respectively). Furthermore, there was no significant difference in PFS although follow-up is short at present (18 months PFS 85% and 84%). There was a lower incidence of AF and haemorrhage in the zanubrutinb arm but a higher incidence of neutropenia.
Acalabrutinib monotherapy was investigated in a single-arm multicentre phase 2 trial recruiting 106 BTKi-naïve patients of whom 92 had relapsed or refractory disease.87 With a median follow-up of 27 months, the 2-year PFS is 82% for those with previously treated disease. Whilst data from the pivotal study of ibrutinib suggested that those rare patients with MYD88WT disease did not benefit from ibrutinib monotherapy, subsequent analysis has shown activity and clinical benefit with both single-agent acalabrutinib and zanubrutinib for those with MYD88WT status.
Other BTKis are currently under investigation, including pirtobrutinib (LOXO-305), a non-covalent BTKi which binds BTK reversibly and has been demonstrated to have clinical benefit even in patients who have progressed on prior BTKi therapy.88
Novel therapies
Venetoclax is a first-in-class, oral, selective B-cell lymphoma-2 (BCL-2) inhibitor and already approved in Europe and the US for the treatment of patients with relapsed/refractory CLL. In a phase I dose-finding study, venetoclax showed efficacy in four WM patients.89 Following this report, a phase II study evaluating the safety and efficacy of venetoclax monotherapy in 32 previously treated WM patients was initiated.90 High levels of response were reported with an ORR of 84% and MRR of 81% with a median PFS of 30 months. Treatment was well tolerated and no clinical tumour lysis syndrome was seen. Grade 3/4 adverse events included neutropenia, anaemia, back pain and constipation.
Novel agents under investigation in WM include checkpoint inhibitor blockers. Pembrolizumab in combination with rituximab is one such agent under investigation (NCT03630042). Given the key role of the MYD88 mutation in WM, compounds that target the BTK–IRAK1/4–NF-κB signalling axis are also in development. The anti-CD38 monoclonal antibody daratumumab exhibited modest single-agent activity in a phase II study.91
Transplantation in WM
The lack of prospective comparative trials makes it challenging to provide high-quality recommendations on the role of stem cell transplant (SCT) in WM. For WM patients who are potential autologous SCT candidates it is important to avoid the use of stem-cell-toxic therapeutic drugs for first-line therapy to reduce risk for stem cell harvest failure.92
Whilst there are case series detailing positive outcomes for autologous SCT (ASCT) for WM as part of first-line therapy, this cannot be recommended outside a clinical trial due to lack of strong evidence, unless there is another indication such as amyloidosis.93-97
In the relapse setting, outcomes with ASCT have been reported in small case series.98-100 The European Bone Marrow Transplant Registry (EBMT) published the outcomes on 158 WM patients with OS and PFS being 68.5% and 39.7% at 5 years respectively.101 More than three prior therapeutic lines, chemorefractory disease and poor performance status were predictors of less favourable outcomes.
An updated EBMT study on 615 WM patients treated with ASCT reported PFS and OS at 5 years of 46% and 65% respectively.102 Relapse incidence was significantly lower when ASCT was performed in the first maximum disease response (CR1, PR1, VGPR1) compared to when the ASCT was used in subsequent complete or partial responses or with refractory disease (39% vs. 53%), translating into a significant PFS (50% vs. 40%) and OS benefit (71% vs. 63%) for the patients transplanted early. ASCT was not beneficial for WM patients who were transplanted with chemorefractory disease or those who had been exposed to more than three line therapies.
The place of allogeneic SCT (alloSCT) in the treatment algorithm of WM has become more controversial especially in the era of new agents even for younger patients.103 There is a high non-relapse mortality (NRM) and the use of alloSCT is therefore limited to highly selected patients. Small retrospective series in heavily pre-treated WM patients suggested that alloSCT was associated with a graft versus WM effect and could prolong PFS and OS in those patients who survived toxicity.99, 100, 104-106 EBMT (n = 86) and the Center for International Blood and Marrow Transplant Research (CIBMTR n = 144) have reported the largest series of WM patients who have undergone allogeneic SCT. Despite a median PFS of approximately 5 years being seen in both cohorts, the NRM seen was high at approximately 30%, although slightly lower for reduced-intensity-conditioned transplants. Patients with chemosensitive disease and better pretransplant disease status experienced significantly superior OS. CAR-T cell therapy is currently experimental and likely to be explored in trials of low-grade lymphomas.
Considerations for choice of therapy
The majority of patients will die of causes not directly related to WM so this is an important factor to consider when deciding on optimal therapy.107 Lack of availability of certain treatment options and a paucity of randomised data between therapies has led to differences in choice of therapies offered to patients worldwide.108 Patients should be offered treatment in clinical trials where available. When considering choice of therapy, the indication for treatment and the speed with which the paraprotein and disease burden needs to be reduced, should be taken into account (Figure 1). For example, rituximab and bendamustine may be favoured over DRC as front-line therapy in patients with amyloidosis causing organ impairment given the shorter time to response with the former regimen. As with all malignancies, patient factors should also be taken into account such as fitness and comorbidities that may be predictive of increased toxicity. Moreover, younger, fitter patients, who may be candidates for autologous SCT as part of future lines of therapy, may benefit from avoidance of stem-cell-toxic drugs.
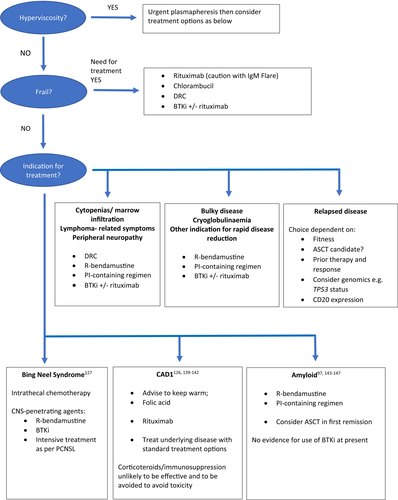
At present there is insufficient evidence to suggest that the mutation status of patients should dictate therapy. There is no definitive evidence from prospective clinical trials that the MYD88 or CXCR4 status is predictive of response to chemoimmunotherapy regimens, although this is complicated by the small numbers of patients in subgroups and also the different methods of assessment which can lead to different sensitivities of detection.109, 110 Prospective data from the US have demonstrated that in patients treated with ibrutinib for relapsed and refractory WM, those with MYD88L265P CXCR4WT had more responses than those with MYD88L265P CXCR4 warts, hypogammaglobulinaemia, immunodeficiency, myelokathexis (WHIM) (97% vs. 68%, p < 0.0001) with no major responses seen in the four patients who were wild-type for both genes.28 Furthermore, there was a suggestion that in the patients with a CXCR4 mutation, nonsense mutations were associated with a poorer outcome. In contrast, the data from iNNOVATE do not demonstrate a difference in outcome based on subgroups differentiated by genotype.29 There is a lack of trial data at present to investigate whether MYD88 or CXCR4 status is predictive of response or PFS with other BTK inhibitors, acalabrutinib or zanubrutinib.85-87, 111 There are data confirming that the MYD88 genotype is also not predictive of response to proteasome inhibitors.34
Although the incidence of TP53 disruption is low in untreated WM patients (2%–10%), this can increase under selective pressure of chemotherapy resulting in chemo-refractoriness. Analogous to CLL, non-chemotherapeutic options should be considered for WM patients harbouring TP53 disruption at relapse.35-37
At relapse, choice of therapy is dependent on previous therapies, time from prior therapy and patient factors. Repeat biopsies where possible are advisable, and confirmation of CD20 expression can be helpful in guiding treatment options as well as repeating genomics such as CXCR4 and TP53 status. In practice, in the UK currently many patients are now considered for BTK inhibitor therapy for relapsed disease, although for younger patients, further chemotherapy and ASCT can be considered for finite treatment length with comparable expected PFS.
Recommendations
Front-line treatment
- Dexamethasone, rituximab and cyclophosphamide (DRC), bendamustine and rituximab (BR), bortezomib regimens (bortezomib, cyclophosphamide and rituximab [BCR] and bortezomib, dexamethasone and rituximab [BDR]) and BTKi are all acceptable front-line treatments (Grade B1).
- Chlorambucil or rituximab monotherapy remain suitable therapy in some elderly frail patients (Grade B1).
- Given the risk of IgM flare, careful monitoring of all patients receiving rituximab is required with monitoring of sequential IgM levels, clinical assessment for hyperviscosity syndrome (HVS) and monitoring of plasma viscosity if available (Grade A1). The introduction of rituximab should be deferred (or prophylactic plasmapheresis performed) in patients considered at a higher risk of hyperviscosity, this being defined by an IgM/ M-protein >40 g/L (Grade C1).
- There is a lack of evidence to support the use of maintenance rituximab at present (Grade B1).
Treatment of relapsed disease
- Treatment with a BTKi, rituximab-containing regimens and bortezomib-containing regimens are options for patients at relapse. Clinical phenotype of the patient is critical in deciding treatment choice (Grade B1).
- Autologous SCT (ASCT) can be considered as a second or later line of therapy in selected chemotherapy-responsive patients but remains contentious in the novel drug era (Grade C2).
- Autologous SCT (ASCT) should not be offered to patients with less than a partial response (PR) (Grade C1).
- Allogeneic SCT should be considered only for highly selected patients who have progressed after immunochemotherapy and BTK inhibitor therapy (Grade C2).
Response assessment
Criteria for the formal assessment of treatment response have been developed and widely applied in WM (Table 2).10 These criteria, based principally on the level of reduction in IgM paraprotein, were proposed to facilitate uniform reporting of clinical trial data but have clinical value in the management of individual patients. There are however a number of caveats that should be considered. Whilst IgM responses appear to predict PFS with rituximab-based conventional therapies, the number of patients achieving a complete response remains very low and clinical benefit may be achieved in some patients who fail to achieve a major IgM response. Whilst a partial response or better after 6 months of ibrutinib was predictive for a longer PFS, deeper responses did not lead to further improvement.112 It should also be noted that response kinetics can vary with different therapies. Rituximab-based chemotherapy combinations may be associated with delayed IgM responses and low incidence of CR, which appears to reflect selective depletion of B cells with persisting plasma cells.113 Repeat marrow assessment has value in the setting of slow/suboptimal response, to exclude for example a discordance in the IgM response to bone marrow response. An expectant approach should be considered if there is B-cell depletion and persistent monotypic plasma cells. Depletion of WM B cells, when assessed with sensitive flow cytometry (limit of detection 0.004%), has recently been shown to be an independent predictor of PFS with rituximab-based therapies.64
| Complete response (CR) |
Absence of serum monoclonal IgM protein by immunofixation Normal serum IgM level Complete resolution of extramedullary disease, i.e., lymphadenopathy and splenomegaly if present at baseline Morphologically normal bone marrow aspirate and trephine biopsy specimen |
| Very good partial response (VGPR) |
Monoclonal IgM protein is detectable ≥90% reduction in serum IgM level from baseline Complete resolution of extramedullary disease, i.e., lymphadenopathy/splenomegaly if present at baseline No new signs or symptoms of active disease |
| Partial response (PR) |
Monoclonal IgM protein is detectable ≥50% but <90% reduction in serum IgM level from baseline Reduction in extramedullary disease, i.e., lymphadenopathy/splenomegaly if present at baseline No new signs or symptoms of active disease |
| Minor response (MR) |
Monoclonal IgM protein is detectable ≥25% but <50% reduction in serum IgM level from baseline No new signs or symptoms of active disease |
| Stable disease (SD) |
Monoclonal IgM protein is detectable <25% reduction and <25% increase in serum IgM level from baseline No progression in extramedullary disease, i.e., lymphadenopathy/splenomegaly No new signs or symptoms of active disease |
| Progressive disease (PD) | ≥25% increase in serum IgM level from lowest nadir (requires confirmation) and/or progression in clinical features attributable to the disease |
Note
- Sequential changes in IgM levels may be determined either by M protein quantitation by densitometry or total serum IgM quantitation by nephelometry.
Recommendations
- Treatment response should be evaluated using uniform response criteria (Grade A1).
- Repeat BM assessment is recommended in patients with suboptimal response, especially to rituximab-based therapies. Evaluation should be at maximal response which can be delayed many months (Grade A1).
- Detailed and systematic evaluation of BM and extramedullary disease should be evaluated in clinical trials (Grade A1).
Investigation and treatment of histological transformation
Histological transformation, primarily to diffuse large B-cell lymphoma (DLBCL), is a well-recognised phenomenon which has been reported to occur in 2.4%–11% of patients with WM.63, 114-122 The largest reported series of 77 patients with secondary DLBCL in patients with WM had a very high incidence of involvement of extranodal sites, seen in 91% of patients including CNS, cutaneous and testicular sites which are all sites where the de novo DLBCL counterpart is strongly associated with MYD88 mutation.121 In this series the median time from diagnosis to high-grade transformation was 4.6 years and 16 patients (21%) had never been treated for WM. Tissue biopsy is essential for a diagnosis of histological transformation and may be directed by PET-CT scanning as has been described in CLL.123
High-grade transformation to DLBCL is treated with similar regimens and approaches used to treat de novo DLBCL, mainly R-CHOP in fitter patients.121 A scoring system based on 133 patients identified three independent predictors of 2-year survival after transformation: elevated serum LDH (two points), platelet count <100 × 109/L (1 point) and any previous treatment for WM (1 point). Three risk groups were defined: low risk (0–1 point, 24% of patients), intermediate risk (2–3 points, 59%, HR = 3.4) and high risk (4 points, 17%, HR = 7.5). Two-year survival rates were 81%, 47%, and 21% respectively (p < 0.0001). This model appeared to be a better discriminant than the International Prognostic Index (IPI) and the revised IPI (R-IPI).124 Consolidation with an autologous SCT may be considered in selected patients responsive to treatment but there is no standard approach.
Recommendations
- Tissue biopsy is required in all patients with suspected histological transformation (Grade A1).
- Tissue biopsy may be directed by PET-CT scanning (Grade A1).
- Treatment of high-grade transformation to DLBCL is with similar regimens used to treat de novo DLBCL but prognosis is less favourable (Grade B1).
- Autologous SCT should be considered as consolidation for high-grade transformation for suitable patients responding to induction chemotherapy (Grade C2).
Complications of WM
WM can affect any system in the body, leading to varying complications which can often be the initial presentation of the disease. The pathogenesis of the complications can either be due to the lymphomatous component of the disease, for example, Bing–Neel syndrome, or due to paraproteinaemia or paraprotein deposition, for example, peripheral neuropathy, or due to specific properties of the paraprotein, for example, CAD.
It is advisable that patients with these complications are investigated and managed in conjunction with appropriate specialists, for example, neurologists/nephrologists, and that tertiary opinions are sought where necessary from haematologists with expertise in WM or amyloidosis.
Although management is specific to each complication, generally most complications are managed through conservative measures, for example, keeping warm for CAD or cryoglobulinaemia; immunosuppression or single-agent rituximab for autoimmune complications such as warm autoimmune haemolytic anaemia (AIHA) or antimyelin-associated glycoprotein (anti-MAG) neuropathy; or by treatment of the WM to reduce the paraprotein/lymphoma—in this case the treatment choice may be guided by the speed by which the paraprotein needs to be reduced and plasmapheresis may be required if urgent reduction of paraprotein is required. International guidelines have been produced for the investigation and management of some of these complications.125-127
The diagnosis of HVS remains a clinical one. The formal assessment of plasma viscosity is useful if there is a concern. Older studies have concluded that it is rare to develop HVS with a plasma or blood viscosity less than 4 milliPascal second (mPas), a more recent retrospective study showed that approximately half of 85 patients with symptomatic HVS had a serum viscosity less that 4 mPas and so it should be considered if greater than 3 mPas.128, 129 Although the relationship between plasma or serum viscosity and IgM level is not linear and differs between patients, hyperviscosity rarely occurs with paraprotein <30 g/L. The likelihood of symptomatic HVS with IgM >60 g/L is controversial, with one paper suggesting median time to HVS of 3 months and another not reaching median time with 9 years follow-up.129, 130 Single-volume plasma exchange will reduce the paraprotein by approximately 40%. It usually takes 1–3 procedures for symptoms and viscosity to reduce.131
Bing–Neel syndrome (BNS) can affect 1% of patients with WM and should be suspected in patients who develop neurological symptoms suggestive of CNS involvement. Furthermore, timing of diagnosis in relation to the diagnosis of WM can also be variable. There is no standardised treatment for BNS, and choice of therapy will partly depend on the patient's symptoms, fitness and comorbidities. The desired goal of therapy should be considered.132 Responses have been seen in patients treated with chemo-immunotherapy regimens given for systemic disease such as rituximab–bendamustine, and also in regimens similar to that given for high-grade CNS disease.127 A international retrospective series has demonstrated the durable efficacy of ibrutinib in 28 patients with BNS and should also be considered.133
Recommendations
- Hyperviscosity is a haematological emergency and is an indication for therapeutic plasma exchange. This is also an indication for definitive treatment (Grade A1).
- Cold agglutinin disease should be managed with conservative measures and if definitive treatment is required, consider single-agent rituximab or rituximab–bendamustine as first-line treatment (Grade B2).
- Organ involvement by AL (IgM-related) amyloid is an indication for definitive treatment, with the aim being to reduce the light chains as quickly and as deeply as possible to minimise end-organ damage. Consider rituximab–bendamustine as first-line treatment in patients considered fit for this regimen (Grade A1).
- Symptomatic cryoglobulinaemia is an indication for definitive treatment. Treatment choice should be guided by severity of symptoms (Grade B1).
- Investigation and management of IgM-related neuropathy should follow International Workshop on Waldenstrom's Macroglobulinaemia (IWWM)-8 consensus guidelines (D’Sa et al., 2017).125
- Investigation and management for Bing–Neel syndrome should follow the guideline by Minnema et al.127
- Collaborative working with other specialists is advised where appropriate, for example, amyloidosis unit, haematologists with an expertise in coagulation, neurologists, nephrologists (Grade A1).
- Clinicians should have a low threshold for investigating symptoms that could represent complications of WM, as some can occur regardless of the level of the paraprotein and may be an indication for definitive treatment (Grade A1).
Supportive care
Infective complications are common in WM, particularly of the respiratory tract, but there is also a lack of data specifically relating to WM. One group noted no cases of Pneumocystis jirovecii pneumonia in 217 patients with WM being treated with ibrutinib despite the low rate of prophylaxis, in contrast to the reported rates in patients with CLL.134 However given the similarities between the two conditions and the dearth of data related to WM specifically, it is reasonable to refer to the BSH guidelines relating to supportive care in CLL.135 These include advice on prophylactic antibiotics, vaccination and immunoglobulin replacement therapy.
IgA and IgG hypogammaglobulinaemia is commonly seen in WM and can persist following treatment. Hypogammaglobulinaemia did not appear to predict the risk of recurrent infection in a retrospective study of 207 untreated patients.9
A large population study demonstrated increased risk of venous thromboembolism (VTE), but not arterial thrombosis, in patients with WM.136 Further prospective data are required before definitive statements on VTE prophylaxis can be made, but these data should be considered when WM patients encounter periods of additional risk, such as surgery.
Conversely, an acquired bleeding diathesis such as von Willebrand's disease can be present in a proportion of patients with WM, and thus bleeding histories and coagulation screening should be considered prior to invasive procedures.137
A proportion of patients with WM have been found to have hepcidin-related iron deficiency anaemia.138 Selected patients with anaemia, low disease burden, and low transferrin saturation (i.e. <10%–12%) unrelated to gastrointestinal bleeding can respond to parenteral iron.
Recommendations
- Selected patients with anaemia, low disease burden, and low transferrin saturation (i.e. <10%–12%) unrelated to gastrointestinal bleeding can respond to parenteral iron (Grade B2).
- Anti-Pneumocystis jirovecii prophylaxis is recommended in patients requiring intensive and/or immunosuppressive treatment including BTKi (Grade B1).
- Anti-herpes simplex virus (HSV) and herpes zoster (HZV) prophylaxis is recommended in patients requiring intensive, immunosuppressive or bortezomib-based therapy (Grade B1).
- All patients with WM should be offered seasonal influenza and SARS-CoV-2 vaccinations (Grade A1).
- All patients with WM should be offered pneumococcal vaccination in the form of pneumococcal conjugate vaccine (PCV13 or Prevenar 13®) followed by pneumococcal polysaccharide vaccine (PPV23 or Pneumovax®), at least 2 months later (UK Department of Health Guidance) (Grade B1).
- Live vaccines, such as polio, herpes zoster and yellow fever, are not recommended. Those patients who are eligible for the shingles vaccine are now able to receive the non-live vaccine, Shingrix® (Grade B1).
- Antimicrobial prophylaxis should be considered for patients with hypogammaglobulinaemia who develop recurrent bacterial infections, according to local antimicrobial protocols. Patients with secondary hypogammaglobulinaemia and recurrent infection despite antimicrobial prophylaxis should be considered for immunoglobulin replacement in accordance with the current UK Department of Health clinical guidelines (Grade B1).
- Patients should be tested for previous viral hepatitis infection prior to each line of therapy, due to the risk of viral reactivation. Prior to commencing treatment, consider discussion with a hepatologist for advice on monitoring and the use of antiviral therapies in those with evidence of current or past infection (Grade B1).
- Patients receiving purine analogues and bendamustine should receive irradiated blood products lifelong (Grade B1).
Management of patients with WM during the coronavirus disease 2019 (COVID-19) pandemic
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). NHS England has categorised all patients with WM as being in the clinically extremely vulnerable category and this has led to shielding, remote monitoring and visitor restrictions. Treatment decisions such as delaying treatment, using less immunosuppressive therapy, reducing the number of cycles or using oral regimens in preference to intravenous treatments can only be made on an individual basis. A great deal of uncertainty remains as to the impact of such decisions. Guidance regarding the timing of vaccination is outlined on the Gov.UK website in The Green Book Immunisation against infectious disease and prompt vaccination is strongly recommended. https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book
DISCLAIMER
Whilst the advice and information in these guidelines is believed to be true and accurate at the time of going to press, neither the authors, the British Society for Haematology nor the publishers accept any legal responsibility of these guidelines. The British Committee for Standards in haematology will review this guideline on an annual basis to ensure it remains up-to-date guidance. Please see the website at www.bcshguidelines.com to find out if the guideline has been updated or amendments added. The website will also show the date the guideline was last reviewed.
ACKNOWLEDGEMENTS
The authors wish to thank Jacky Wilson for help in undertaking the initial literature review. The authors would like to thank the BSH Haemato-Oncology task force, the BSH sounding board, and the BSH guidelines committee for their support in preparing this guideline.
DECLARATION OF INTERESTS
The BSH paid the expenses incurred during the writing of this guideline. All authors have made a declaration of interest to the BSH and Task Force Chairs which may be reviewed on request.




