Distinct maternal and fetal pregnancy outcomes in women with sickle cell disease can be predicted using routine clinical and laboratory data
A. K. M. and K. H. M. K. contributed equally and share first authorship.
Presented in abstract form at the 61st Annual Meeting and Exposition of the American Society of Hematology, Orlando, FL, December 2019.
Summary
We aimed to identify risk factors for adverse outcomes in pregnancies of women with sickle cell disease (SCD) and develop risk prediction models. Models were derived from a retrospective cohort of pregnant women with SCD and constructed using generalised estimating equation logistic regression, with clustering by woman. Maternal event(s) consisted of acute anaemia; cardiac, pulmonary, hepatobiliary, musculoskeletal, skin, splenic, neurological or renal complications, multi-organ failure, venous thromboembolism, admission-requiring vaso-occlusive events (VOE), red cell transfusion, mortality or hypertensive disorder of pregnancy. Fetal events included preterm birth, small-for-gestational-age or perinatal mortality. Of 199 pregnancies, 71% and 45% resulted in adverse maternal and fetal outcomes respectively. Low first-trimester haemoglobin, admission-requiring VOE in the year before pregnancy, multiple transfusions before pregnancy, SCD genotype and previous cardiac complications predicted maternal risk. Younger age and SCD genotype allowed early prediction of fetal risk (model-F1). Adding maternal event(s) and high lactate dehydrogenase enabled re-assessment of fetal risk with advancing gestation (model-F2). Models were well calibrated and moderately discriminative for maternal outcome (c-statistic 0·81, cross-validated value 0·79) and fetal outcome (model-F1 c-statistic 0·68, cross-validated value 0·65; model-F2 c-statistic 0·72, cross-validated value 0·68). The models will allow early identification of women with SCD at high risk of adverse events, permitting early targeted interventions and ongoing fetal risk re-assessment enabling intensification of surveillance and optimisation of delivery timing.
Introduction
Sickle cell disease (SCD) is a common haemoglobinopathy, accounting for 28 600 deaths globally.1 Red cell deformation into rigid, sickle shapes under hypoxic stress conditions leads to ischaemia–reperfusion injury, haemolysis and endotheliopathy, resulting in organ damage and premature mortality.2, 3 While pregnancy in women with SCD is currently viewed more favourably,4 maternal–fetal morbidity and mortality persist.5-9
Sickle cell disease predominantly affects Black women. Given recently highlighted racial disparities in maternal mortality rates, three-times higher for African American in comparison to Caucasian women in the USA10 and nearly five-times higher for Black compared to White women in the UK,11 the possible impact this condition has on this marginalised population, including curtailed life-achievement potential and increased healthcare utilisation cannot be underestimated.
Studies predicting pregnancy-related complications in women with SCD are lacking, while available interventions carry inherent risks. A meta-analysis comparing prophylactic to on-demand transfusion demonstrated reduction of adverse pregnancy outcomes with the former; limited by low-quality studies and scant trial data.12 A subsequent investigation of early prophylactic erythrocytapheresis garnered further support for its benefits.13 However, given transfusion-related complications,14 its non-judicious use is undesirable. Identification of risk factors for adverse pregnancy outcomes would enable early consideration of therapeutic interventions for those poised to derive utmost benefit, whilst shielding those at low risk of potential treatment-associated harms. It may also permit intensification of fetal surveillance or delivery planning in those at higher risk of fetal events. The purpose of the present study was to identify risk factors associated with adverse pregnancy outcomes in women with SCD, to develop and internally validate prediction models capable of distinguishing pregnancies at higher risk of adverse outcomes.
Methods
This is a retrospective cohort study of all pregnant women with SCD, treated and delivered at Mount Sinai Hospital in Toronto, a quaternary centre for pregnant patients with SCD (1 January 1990 to 31 December 2016), affiliated with the University of Toronto, supporting the largest Maternal-Fetal Medicine Division in Canada, with 7800 births annually. Research Ethics Board approval was obtained (13-0171-C).
Sickle cell disease was established by haemoglobin electrophoresis, with confirmation by genetic analysis where diagnosis was unclear. Individuals with HbSS and HbS/β0-thalassaemia were analysed together, as were individuals with HbSC and HbS/β+-thalassaemia, in keeping with previous studies.15 Adverse maternal and fetal events were those first noted during the pregnancy within the study period (hereafter called study-pregnancy) (Table I). For twin pregnancies, adverse fetal events were considered present if either infant met criteria.
| Composite of adverse maternal events | Composite of adverse fetal events | ||
|---|---|---|---|
| Outcome | Definition | Outcome | Definition |
| Acute anaemia |
Hyperhaemolysis Acute splenic sequestration Aplastic crises |
PTB | Delivery <37 weeks’ GA |
| Cardiac complication |
Congestive heart failure Cardiomyopathy Cardiomegaly |
SGA | Birthweight <10th percentile for GA |
| Pulmonary complication |
Acute chest syndrome Pulmonary hypertension Pneumonia |
Perinatal mortality | Absence of fetal heart rate confirmed by ultrasonography >12 weeks’ GA or neonatal death (infant's death prior to discharge from hospital) |
| Hepatobiliary complication | Intrahepatic cholestasis | ||
| MSK/skin complication |
Myositis/fasciitis Osteomyelitis Abscess Inflammatory tissue masses |
||
| Splenic complication |
Infarction Hypersplenism |
||
| Neurological complication* |
TIA Stroke |
||
| Renal complication |
Acute renal failure Pyelonephritis Recurrent urinary tract infections Proteinuria† |
||
| Multi-organ failure | |||
| Venous thromboembolism |
DVT PE |
||
| VOE requiring admission | |||
| RBC transfusion | |||
| Maternal mortality | |||
| Hypertensive disorder of pregnancy |
Gestational hypertension Pre-eclampsia |
||
- SCD-associated factors are defined based on published classification of phenotypic manifestations of SCD.16 Hypertensive disorders of pregnancy are categorised as gestational hypertension (blood pressure >140/90 mmHg identified in pregnancy, without proteinuria), or pre-eclampsia (blood pressure >140/90 mmHg, new onset/worsening proteinuria, or adverse conditions/complications),36 as noted in health records. SGA size is defined as birthweight (BW) <10th percentile for GA per population-based Canadian reference,37 and perinatal mortality as ultrasonography confirmed absence of fetal heart rate after 12 weeks’ gestation, or neonatal death prior to discharge from hospital. DVT, deep vein thrombosis; GA, gestational age; MSK, musculoskeletal; PE, pulmonary embolism; PTB, preterm birth; RBC, red blood cell; SCD, sickle cell disease; SGA, small for gestational age size; TIA, transient ischaemic attack; VOE, vaso-occlusive events.
- * As noted in the health record.
- † Only if first identified during study-pregnancy and outside the context of a hypertensive disorders of pregnancy.
For a factor to be considered a potential adverse outcome predictor, its presence before onset of the outcome was ascertained, as was its absence from the outcome’s definition. Predictor variables included sickle-cell genotype; maternal age; parity; maternal weight; pregnancy-associated weight gain; body mass index (BMI); pre-pregnancy hydroxyurea use; prophylactic (simple or exchange) red blood cell (RBC) transfusion before pregnancy;12 RBC transfusions newly initiated during study-pregnancy; first-trimester white blood cell counts, platelet counts and haemoglobin levels and highest level of lactate dehydrogenase (LDH) any time in pregnancy. LDH was considered high upon exceeding two standard deviations (SDs) above the upper normal range (135–225 u/l), and normal when under two SDs of the upper normal range or when not completed (given typically drawn when haemolysis suspected). Phenotypic manifestations of SCD, listed and defined in Table II, and categorised according to published definitions,16 were also considered as potential predictors. Within this group, chronic processes (i.e. avascular necrosis) were considered as potential predictors when identified before pregnancy or during study-pregnancy, while acute or episodic events were considered as potential predictors, solely when identified before pregnancy and absent during study-pregnancy.
| Category | Diagnosis | Before pregnancy | Study-pregnancy |
|---|---|---|---|
| Acute anaemia |
Hyperhaemolysis Acute splenic sequestration Aplastic crises |
X | |
| Cardiac events |
Congestive heart failure Cardiomyopathy Cardiomegaly Hypertension |
X | |
| Pulmonary events |
Acute chest syndrome* Pneumonia Pulmonary hypertension* |
X | X* (excluding pneumonia) |
| Hepatobiliary events |
Cholecystitis* Cholelithiasis* Hepatic sequestration* Viral hepatitis* Intrahepatic cholestasis |
X | X* (excluding intrahepatic cholestasis) |
| Muscular/skeletal/skin events |
Osteomyelitis* Avascular necrosis* Dactylitis* Myositis/fasciitis Leg ulcers |
X | X (excluding myositis/fasciitis and leg ulcers) |
| Neurological events |
Transient ischaemic attack Stroke |
X | |
| Ophthalmological events |
Glaucoma Sickle cell retinopathy Vitreous haemorrhage Retinal detachment |
X | X |
| Renal events |
Acute renal failure Proteinuria/nephrotic syndrome Pyelonephritis Chronic renal failure* Haematuria* |
X |
X Only chronic renal failure and haematuria |
| VTE |
DVT PE |
X | |
| Vaso-occlusive events (VOE) | Considered a predictor only if it required hospitalisation and occurred in the year preceding the study-pregnancy | X |
- DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
- * If first identified or present acutely during study-pregnancy.
A rational, pragmatic approach guided selection of potential predictors in this dataset, allowing for clear distinction between those that did and did not meet criteria for inclusion in the model. Among variables pre-specified as potential predictors, those that exhibited insufficient variability between pregnancies and had >10% missing values were excluded. Remaining variables were compared between pregnancies with and without adverse outcomes to determine suitability for inclusion in the regression model. Counts and percentages were used to summarise categorical variables, and means and SDs for continuous variables, with the exception of platelets, where the median and interquartile range were used. Pregnancies with and without adverse outcomes were compared with chi-squared tests or Fisher’s exact tests for categorical variables (according to whether the overall percentages in categories were all >10%) and with t-tests for continuous variables (with the exception of platelets, which used the Wilcoxon rank-sum test). Potential clustering by repeated pregnancies was not accounted for in these mainly descriptive analyses, where the univariate P values are presented as an aid to identification of variables that may differ according to adverse outcomes. Separate regression models for adverse maternal and fetal outcomes were constructed. Missing values for weight gain from booking to 32 weeks, first-trimester haemoglobin levels and booking-visit BMI were replaced in a single imputation procedure using the ‘mice’ package.17 To account for non-independence of outcomes in women with multiple study pregnancies in the regression models, univariate generalised estimation equation (GEE) logistic regression with clustering by woman was used to calculate odds ratios for potential predictors (Table SI).
A maternal model was developed, using variables available during the first trimester, as early prediction of adverse outcomes was desired to identify pregnancies most likely to derive treatment benefits. Two fetal models were created: model-F1, with first-trimester variables, reflecting the aim of early prediction described for the maternal model and model-F2 including variables evolving during pregnancy, for ongoing re-assessment throughout gestation, permitting intensification of fetal surveillance or optimisation of delivery-planning with high risk of fetal events. Fetal and maternal risk calculators were created.
Those predictors with univariate P < 0·2 were included in a multivariable GEE regression model subject to the rule that ˜10 events and non-events are needed per modelled parameter.18 Discriminative performance of the fitted model (ability to distinguish those who will experience an adverse maternal or fetal event) was assessed using the concordance statistic and its accuracy of prediction was evaluated with a calibration curve. A 10-fold cross-validation was used to estimate the out-of-sample performance of the regression models with the selected variables. All analyses were completed using R 3.5.119 by G.T. All authors had access to the data.
Results
Analysis included 199 pregnancies in 131 women, with 77, 41, 12 and one of the women contributing one, two, three and four pregnancies respectively. HbSS or HbS/β0-thalassaemia was present in 76 (58%) women. There was no maternal mortality and perinatal mortality was seen in nine (4·5%) pregnancies, with no neonatal deaths. Table III provides demographics and univariate analysis of potential predictors.
| Characteristics | Overall cohort (n = 199) | Composite of adverse maternal events | Composite of adverse fetal events | ||||
|---|---|---|---|---|---|---|---|
| Present (n = 142) | Absent (n = 57) | P | Present (n = 95) | Absent (n = 104) | P | ||
| Year of delivery, n (%) | |||||||
| 1991–2004 | 55 (27·6) | 47 (33·1) | 8 (14·0) | 0·014 | 27 (28·4) | 28 (26·9) | 0·226 |
| 2005–2009 | 47 (23·6) | 31 (21·8) | 16 (28·1) | 19 (20·0) | 28 (26·9) | ||
| 2010–2014 | 47 (23·6) | 27 (19·0) | 20 (35·1) | 28 (29·5) | 19 (18·3) | ||
| 2015–2017 | 50 (25·1) | 37 (26·1) | 13 (22·8) | 21 (22·1) | 29 (27·9) | ||
| Maternal age, years, mean (SD) | 27·4 (5·3) | 27·3 (5·2) | 27·8 (5·7) | 0·49 | 26·3 (5·0) | 28·4 (5·5) | 0·006 |
| HbSS or HbS/β0-thalassaemia, n (%) | 111 (55·8) | 96 (67·6) | 15 (26·3) | <0·001 | 66 (69·5) | 45 (43·3) | <0·001 |
| Multiparity, n (%) | 101 (50·8) | 73 (51·4) | 28 (49·1) | 0·89 | 45 (47·4) | 56 (53·8) | 0·44 |
| BMI, kg/m2, mean (SD) | 24·6 (4·6) | 24·0 (4·5) | 26·1 (4·7) | 0·003 | 23·8 (4·5) | 25·3 (4·6) | 0·02 |
| Maternal weight gain by 32 weeks*, kg, mean (SD) | 5·2 (4·6) | 5·2 (4·1) | 5·3 (5·7) | 0·92 | 4·8 (3·9) | 5·6 (5·1) | 0·25 |
| Hb T1, g, mean (SD) | 91 (16·4) | 88 (16·3) | 100 (13·3) | <0·001 | 87 (17·7) | 94 (14·3) | 0·002 |
| WBC T1†, ×109/l, mean (SD) | 11·0 (3·7) | 11·4 (3·6) | 9·6 (3·7) | 0·009 | 11·2 (4·0) | 10·7 (3·4) | 0·42 |
| Platelets T1‡,×109/l, median (IQR) | 316 (192–398) | 335 (235–401) | 232 (150–363) | 0·03 | 312 (201–397) | 318 (184–388) | 0·77 |
| Previous adverse maternal event(s), n (%) | 108 (54·3) | 88 (62·0) | 20 (35·1) | 0·001 | 54 (56·8) | 54 (51·9) | 0·57 |
| Adverse maternal event(s) in study-pregnancy, n (%) | – | – | – | – | 80 (84) | 62 (60) | <0·001 |
| Acute anaemia before pregnancy§, n (%) | 8 (4·0) | 6 (4·2) | 2 (3·5) | 1·00 | 3 (3·2) | 5 (4·8) | 0·72 |
| Cardiac before pregnancy, n (%) | 27 (13·6) | 24 (16·9) | 3 (5·3) | 0·05§ | 14 (14·7) | 13 (12·5) | 0·68§ |
| Pulmonary before pregnancy, n (%) | 74 (37·2) | 60 (42·3) | 14 (24·6) | 0·03§ | 35 (36·8) | 39 (37·5) | 1·00§ |
| Multi-organ failure before pregnancy§, n (%) | 1 (0·5) | 1 (0·7) | 0 (0·0) | 1·00 | 1 (1·1) | 0 (0·0) | 0·48 |
| Hepatobiliary before pregnancy§, n (%) | 12 (6·0) | 11 (7·7) | 1 (1·8) | 0·19 | 5 (5·3) | 7 (6·7) | 0·77 |
| Neurological before pregnancy§, n (%) | 4 (2·0) | 4 (2·8) | 0 (0·0) | 0·58 | 3 (3·2) | 1 (1·0) | 0·35 |
| Muscular/skin/skeletal before pregnancy, n (%) | 35 (17·6) | 31 (21·8) | 4 (7·0) | 0·02 | 20 (21·1) | 15 (14·4) | 0·30 |
| Ophthalmological before pregnancy§, n (%) | 15 (7·5) | 11 (7·7) | 4 (7·0) | 1·00 | 9 (9·5) | 6 (5·8) | 0·42 |
| Renal before pregnancy§, n (%) | 12 (6·0) | 9 (6·3) | 3 (5·3) | 1·00 | 7 (7·4) | 5 (4·8) | 0·56 |
| VTE before pregnancy§, n (%) | 6 (3·0) | 6 (4·2) | 0 (0·0) | 0·19 | 1 (1·1) | 5 (4·8) | 0·22 |
| VOE¶ before pregnancy, n (%) | 62 (31·2) | 56 (39·4) | 6 (10·5) | <0·001 | 33 (34·7) | 29 (27·9) | 0·37 |
| Acute chest syndrome before pregnancy, n (%) | 25 (12·6) | 25 (17·6) | 0 (0·0) | 0·002 | 15 (15·8) | 10 (9·6) | 0·27 |
| Multiple transfusion** before pregnancy, n (%) | 133 (66·8) | 105 (73·9) | 28 (49·1) | 0·001 | 66 (69·5) | 67 (64·4) | 0·55 |
| High LDH in study-pregnancy, n (%) | 75 (37·7) | 67 (47·2) | 8 (14·0) | <0·001 | 48 (50·5) | 27 (26·0) | 0·001§ |
| Highest LDH** in study-pregnancy, u/l, mean (SD) | 580 (450) | 645 (480) | 341 (169) | 0·002 | 694 (497) | 450 (350) | 0·002 |
| Hydroxyurea before pregnancy, n (%) | 37 (18·6) | 34 (23·9) | 3 (5·3) | 0·004 | 22 (23·2) | 15 (14·4) | 0·16 |
| Previous adverse fetal event(s)**, n (%) | – | – | – | – | 23 (24) | 16 (15) | 0·17 |
| Birth weight, g, mean (SD) | 2745 (716) | 2646 (724) | 2988 (637) | 0·002 | N/A as individual parameters are part of adverse fetal events | ||
| SGA, n (%) | 57 (29·1) | 47 (33·8) | 10 (17·5) | 0·04 | |||
| Gestational age at delivery, weeks, mean (SD) | 37·3 (3·7) | 36·9 (3·9) | 38·3 (3·2) | 0·012 | |||
| PTB <37 weeks, n (%) | 52 (26·1) | 47 (33·1) | 5 (8·8) | 0·001 | |||
- Comparisons between groups do not account for potential clustering induced by repeated pregnancies in the same woman. P values for continuous variables were computed from two-sample t-tests, with the exception of the P value for platelets, which used the Wilcoxon rank-sum test. P values for categorical variables come from chi-squared tests, unless the overall frequency of a category was <10%, in which case Fisher’s exact test was used. BMI, body mass index; Hb T1, haemoglobin in the first trimester; IQR, interquartile range; LDH, lactate dehydrogenase; PTB, preterm birth; SGA, small for gestational age; SD, standard deviation; VOE, vaso-occlusive events; VTE, venous thromboembolism; WBC T1, white blood cell count in the first-trimester.
- * No weights available for nine pregnancies, thus analysis based on 190 pregnancies.
- † WBC measurement was missing in 59 pregnancies, thus not advisable to include this as a predictor in the main model, but association of WBC with adverse maternal events in patients where measurements were available was assessed by adding this variable to the regression model [odds ratio (OR) 1·053, 95% confidence interval (CI) 0·93–1·20; P = 0·4306].
- ‡ Platelet count levels were missing in 58 pregnancies, thus not advisable to include as a predictor in the main model, but association of WBC with adverse maternal events in patients where measures were available was assessed by adding this variable to the regression model (OR 1·0, 95% CI 0·997–1·003; P = 0·9626).
- § Fisher’s exact.
- ¶ VOE requiring admission in the year preceding the pregnancy within the study period; data available for 139 pregnancies with adverse maternal events, 55 pregnancies with no adverse maternal events, 81 pregnancies with adverse fetal events and 85 pregnancies with no adverse fetal events.
- ** Transfusions defined as five or more distinct time periods when transfusion was administered before pregnancy.
- †† LDH defined as two SDs above upper range of normal (135–225 u/l), data available for 96 pregnancies with adverse maternal events 26 pregnancies with no adverse maternal events, 65 pregnancies with adverse fetal events and 57 pregnancies with no adverse fetal events.
- ‡‡ History of adverse fetal events was not suitable for inclusion in the model, as the information was not available for 130 pregnancies which occurred outside of the study period.
Maternal outcome predictors
Adverse maternal events were encountered in 142 (71%) pregnancies; with a similar frequency in first (69/98, 70%) and subsequent pregnancies (73/101, 72%) (Table III). Within the latter group, incidence was higher when adverse maternal events were experienced in an earlier pregnancy (57/66, 86%) than when they were not (16/35, 46%; P < 0·001). Prior hydroxyurea use at any time was documented in 37 (19%) pregnancies: 27 solely before conception and 10 extending into the first trimester. Prior hydroxyurea use was more common in pregnancies with than without adverse maternal events (34/142, 24% vs. three of 57, 5%); suggesting it may be a marker of severity, rather than an independent risk factor for adverse maternal events.
Table III summarises results for the uni- and multivariable GEE models. Risk of adverse maternal events increased with lower first-trimester haemoglobin levels, vaso-occlusive events (VOE) requiring admission in the year preceding the study-pregnancy, multiple transfusions before pregnancy, SCD genotype group HbSS/HbSβ0-thalassaemia and cardiac complications before pregnancy.
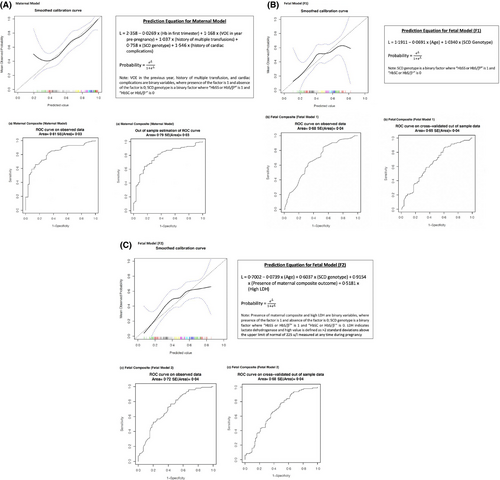
The multivariable GEE model was strongly discriminative for occurrence of adverse maternal events, with a concordance statistic of 0·81 (SE = 0·03) on the observed data and a cross-validated value of 0·79 (SE = 0·03). Figure 1 includes the prediction equation and receiver operating characteristic (ROC) curves; with the risk calculator here:16 https://tomlinson-bru.shinyapps.io/SCDPregnancyOutcomes/
Fetal outcome predictors
Adverse fetal events were observed in 95 (48%) pregnancies. The influence of a previous adverse fetal event(s) on subsequent pregnancies was examined in a subset of 68 women for whom all prior pregnancies were captured. Those with and without a previous adverse fetal event had event rates of 59% and 28% respectively (P = 0·04). There was no relationship between maternal hydroxyurea use at any time and occurrence of adverse fetal events. Furthermore, no fetal anomalies were identified in the 10 pregnancies with first-trimester hydroxyurea exposure.
Table III details cohort characteristics according to presence or absence of adverse fetal event(s). Table IV summarises results for the uni- and multivariable GEE models. In model-F1, risk of adverse fetal event(s) was higher with younger maternal age and SCD genotype group HbSS/HbSβ0-thalassaemia. In model-F2, risk of adverse fetal event(s) was higher with younger maternal age, SCD genotype group HbSS/HbSβ0-thalassaemia, occurrence of adverse maternal events and/or high LDH.
| Predictor | Univariate analyses | Multivariable analyses | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Characteristics predictive of adverse maternal events (present in early pregnancy) | |||||
| Hb T1 (g/l), per 15 unit increments | 0·51 (0·35–0·72) | <0·001 | 0·67 (0·44–1·02) | 0·06 | |
| VOE* before pregnancy, n (%) | 3·84 (1·68–8·76) | 0·001 | 3·22 (1·25–8·24) | 0·02 | |
| Multiple transfusion† before pregnancy, n (%) | 4·93 (2·09–11·64) | <0·001 | 2·82 (1·05–7·55) | 0·04 | |
| HbSS or HbS/β0-thalassaemia, n (%) | 5·23 (2·48–11·0) | <0·001 | 2·13 (0·86–5·28) | 0·10 | |
| Cardiac complications before pregnancy, n (%) | 3·37 (1·18–9·61) | 0·02 | 4·69 (1·53–14·41) | 0·01 | |
| Characteristics predictive of adverse fetal events (present in early pregnancy: model-F1) | |||||
| Maternal age (years), per 5 unit increments | 0·73 (0·56–0·93) | 0·01 | 0·71 (0·55–0·91) | 0·01 | |
| HbSS or HbS/β0-thalassaemia, n (%) | 2·97 (1·55–5·67) | 0·001 | 2·81 (1·49–5·31) | 0·001 | |
| Characteristics predictive of adverse fetal events (potentially evolving through pregnancy: model-F2) | |||||
| Maternal age (years), per 5 unit increments | 0·73 (0·56–0·93) | 0·01 | 0·69 (0·53–0·91) | 0·01 | |
| HbSS or HbS/β0-thalassaemia, n (%) | 2·97 (1·55–5·67) | 0·001 | 1·83 (0·91–3·66) | 0·09 | |
| High LDH in study-pregnancy, n (%) | 2·36 (1·34–4·16) | 0·003 | 1·68 (0·88–3·19) | 0·11 | |
| Adverse maternal event(s), n (%) | 2·96 (1·44–6·11) | 0·003 | 2·50 (1·13–5·53) | 0·02 | |
- BMI, body mass index; Hb T1, haemoglobin in the first-trimester; LDH, lactate dehydrogenase; SD, standard deviation; VOE, vaso-occlusive events.
- * VOE requiring admission in the year preceding the pregnancy within the study period.
- † Transfusions defined as five or more distinct time periods when transfusion was administered before pregnancy.
Model-F1 was moderately discriminative for occurrence of adverse fetal event(s), with a concordance statistic of 0·68 (SE = 0·04) on observed data and a cross-validated value of 0·65 (SE = 0·04). Model-F2 was more strongly discriminative for occurrence of adverse fetal event(s), with a concordance statistic of 0·72 (SE = 0·04) on observed data and a cross-validated value of 0·68 (SE = 0·04). (Fig 1b and 1c, including prediction equations and ROC curves; fetal risk calculators here): https://tomlinson-bru.shinyapps.io/SCDPregnancyOutcomes/.
Discussion
In the present large SCD cohort, adverse maternal and fetal events occurred in 71% and 45% of pregnancies respectively. Low first-trimester haemoglobin levels, admission-requiring VOE in the year before pregnancy, multiple transfusions before pregnancy, HbSS/HbSβ0-thalassaemia genotype and previous cardiac complications predicted maternal risk of an adverse pregnancy event. Younger maternal age and HbSS/HbSβ0-thalassaemia genotype allowed prediction of fetal risk earlier in gestation, while incorporating occurrence of adverse maternal events and elevated LDH predicted fetal risk with advancing gestation. The maternal model was strongly discriminative, while both fetal models were moderately discriminative for adverse maternal and fetal events respectively.
Consistent with previous investigations,5, 7-9, 20 our present study demonstrated that despite contemporary management, most pregnancies in women with SCD are affected by an adverse outcome; validating the need, whenever feasible, for management of pregnant women with SCD by maternal-fetal medicine physicians in close collaboration with haematologists, in centres with expertise.
Our present analysis revealed that parameters predicting maternal events are not necessarily the same as those predicting fetal events. It became apparent that assessment of maternal and fetal risk would benefit from distinct prediction models. With respect to the utility of the models for clinical practice, two aims emerged: (i) early identification of adverse maternal and/or fetal event risk facilitating consideration of treatment, such as transfusion; and (ii) ongoing fetal-risk evaluation allowing for tailored fetal surveillance and/or decision-making regarding delivery timing. Consequently, the maternal model and first fetal model-F1 contain parameters available in the first trimester [fulfilling aim (i)], while the second fetal model-F2 incorporates additional variables, present later in gestation [fulfilling aim (ii)].
The focus of the first objective was confined to early pregnancy based on our meta-analysis suggesting that prophylactic (compared to on-demand) transfusion was associated with reduction in maternal mortality, VOE and pulmonary complications; with no impact on rates of pre-eclampsia or small-for-gestational-age/low-birth-weight infants, stemming from placental insufficiency, the amelioration of which requires treatment in early gestation to optimise placental development.12 This premise was supported by a subsequent demonstration that prophylactic erythrocytapheresis, initiated in the first trimester, can significantly improve maternal and fetal outcomes.13
Our rationale for risk stratification of the individuals most likely to benefit from prophylactic transfusion, emanated from the recognition that up to 30% of women with SCD will have an uneventful pregnancy. Restriction of prophylactic transfusion to the group identified by the model as at-risk of adverse maternal or fetal events would shield the lower-risk group from potential transfusion-related complications.14
The utility of the second fetal model rests on the premise that even when maternal conditions are optimised, the risk of adverse events is not eliminated completely. As such, an adverse maternal event or high LDH in later gestation allows for re-stratification of fetal risk; and while institution of maternal intervention may no longer be of benefit, the added knowledge can inform decision-making regarding fetal surveillance and delivery planning.
In our present study, adverse maternal events occurred with similar frequency in first as in subsequent pregnancies, although within the latter group, their incidence was higher with a prior adverse event. Similarly, in the subset of women for whom every pregnancy was captured, those with a previous adverse fetal event were more likely to encounter a recurrence. While history of prior adverse maternal or fetal events was not suitable for inclusion in the models, as it would not apply to primiparous women, it should be considered as a further marker of risk.
Supporting prior findings that frequency of admissions in the year before pregnancy were associated with a higher likelihood of on-demand transfusion during pregnancy,21 we also ascertained that an admission-requiring VOE within the year before pregnancy was an independent risk factor for adverse maternal events. Whilst the effect of transfusions on maternal and fetal outcomes has been investigated by many,12, 13, 21, 22 our present study is the first to highlight a multiple-transfusion history as a predictor of adverse pregnancy outcomes.
While it is recognised that systolic and diastolic function parameters in pregnancy differ between women with and without SCD,23 and that impaired cardiac function is observed in 15% of pregnant women with SCD,24 our observation of a link between cardiac complications and morbidity in women with SCD has not been described previously. It is notable, in that many of these women may be asymptomatic at the onset of pregnancy, yet would benefit from cardiac assessment and optimisation, and perhaps echocardiography surveillance with advancing gestation.
The association of HbSS/HbSβ0-thalassaemia with a higher likelihood of adverse maternal events in our model parallels previous studies indicating a greater probability of anaemia, transfusion, VOE, intensive care unit admission and caesarean delivery in HbSS individuals.8, 20 Our present fetal model likewise revealed a relationship between SCD genotype and adverse fetal events, echoing results of a systematic review demonstrating significantly higher odds of adverse fetal events for women with HbSS in comparison to women with HbSC/unspecified SCD.7 Yet, while risks of unfavourable pregnancy outcomes are greater with HbSS/HbSβ0-thalassaemia than with HbSC/HbSβ+-thalassaemia, the latter group does remain at higher risk of adverse events compared to the general obstetric population and, whenever feasible, should be managed in a centre with expertise.7
Our present finding of the impact of low first-trimester haemoglobin on maternal complications in women with SCD is novel. Whilst first-trimester anaemia in the non-SCD population has been linked with adverse pregnancy outcomes,25, 26 its aetiology in those cohorts was predominantly nutritional, whereas effects of anaemia in SCD are primarily mediated by haemolysis. In keeping with this premise, high LDH anytime in pregnancy was significantly associated with adverse fetal events in our present study. Similarly, high LDH was predictive of adverse maternal events in our original maternal model (Figure S1); yet could not be retained in the final model given its aim of early recognition, and the scarcity of LDH determinations in early pregnancy within our dataset. Thus, the notion of the haemolytic SCD-phenotype, which mediates the degree of anaemia and presents more frequently in women with HbSS or HbS/β0-thalassaemia,27, 28 as the driver of adverse maternal and fetal events, may deserve exploration.
The contribution of younger maternal age to the risk of adverse fetal events was unexpected. Whereas some studies in women with SCD adjusted for maternal age, none addressed its effect on pregnancy outcomes.29, 30 Furthermore, the only relevant data in the non-SCD pregnant population concerns adolescent pregnancies, in which adverse fetal events are linked to poor nutrition and low socioeconomic status.31 While the SCD population is at higher risk of these influences, it is conceivable that younger women with SCD are disproportionately affected. Another explanation may lie in the fact that those women who have complicated pregnancies in their younger years ages choose not to have children as they age, whereas those who have a less complicated disease course may delay childbearing, or may have more children as they age if their initial pregnancies were uneventful.
The fetal impact of adverse maternal events has been documented in critically ill pregnant women,32 with 10% of fetal deaths linked to chronic maternal disease.33 This is perhaps unsurprising, as fetal well-being relies on intact maternal physiological adaptations, which may become taxed by acute exacerbations of chronic disease. Our present study explicitly links occurrence of a maternal event during an index pregnancy with higher risk of adverse fetal events. Given that adverse maternal events typically occur later in pregnancy, fetal model-F2 enables re-assessment of fetal risk with advancing gestation.
Our present study is limited by sample size and single-centre retrospective design, potentially influencing generalisability. However, most patients with SCD in North America, the UK, and Europe are managed in comprehensive care centres, and our study-outcomes parallel those cohorts,20 suggesting that our models can be generalised. While our models underwent internal validation, they will benefit from external validation in other cohorts. The time frame of our study is lengthy; yet its findings remain applicable, as it has been shown that pregnancy outcomes in women with SCD have remained unchanged since at least the 1980s,34 further highlighting the need for novel and creative approaches to risk stratification and treatment. Sensitivity analyses (not shown) found that the relationship between predicted risk and actual risk did not vary significantly over the year of delivery for the maternal composite outcome, but that this relationship did vary by year for the two-variable model for the fetal outcome; the residual variation by year is reflected in the lower discriminative ability of the model for the fetal outcome.
Study strengths include the comprehensive evaluation of multiple risk factors and outcomes, and robust statistical analyses including GEE modelling, accounting for potential clustering introduced by multiple pregnancies from a single woman. The models show robust discriminative ability to predict pregnancies at moderate risk of adverse outcomes. Despite the inclusion of a large and diverse set of variables, none have the capacity to successfully identify those at very high and very low risk of complications, raising the possibility of an as yet unexplored factor with significant predictive ability, the identification of which may lead to amendment of the proposed models.
Sickle cell disease persists as a significant issue in women’s health, disproportionately affecting African American women. Identification of sub-groups of women with SCD at high risk of adverse events will enable consideration of early interventions to improve pregnancy outcomes. The ongoing ability to re-assess fetal risk will permit establishment of closer fetal surveillance or allow exploration of delivery timing in response to fetal risk. To facilitate achievement of this goal, a paradigm shift is vital, from continued focus on observational studies to prospective investigations and intervention trials evaluating treatment efficacy. Our present prediction models stand well-poised to allow for risk stratification and adaptation of interventions to improve maternal and fetal outcomes in this condition. Further, they can serve to identify women who may benefit from recruitment to trials. This parallels calls for inclusion of pregnant women in relevant trials35 and is supported by recent United States Food and Drug Administration (FDA) guidance.36
In conclusion, our present data indicate that most pregnancies in women with SCD are affected by an adverse maternal event, while an adverse fetal event is encountered in almost half. Risk of the former can be predicted by presence of low first-trimester haemoglobin, admission-requiring VOE in the year before pregnancy, multiple transfusions before pregnancy, HbSS/HbSβ0-thalassaemia genotype and history of maternal cardiac complications. Risk of the latter in early pregnancy can be predicted by younger maternal age and HbSS/HbSβ0-thalassaemia genotype and can be adjusted as the pregnancy advances by factoring in presence of adverse maternal events and high LDH. We have developed risk calculators that can aid clinical judgement, may be used to externally validate our findings and can be utilised in the development of research protocols for management of pregnant women with SCD.
Acknowledgements
There was no funding for this study. The authors wish to thank Ryan Seeto and Sidra Shafique, research assistants, for their contributions to the initial data collection.
Conflict of interest
A. Kinga Malinowski: Honorarium, Advisory Board – Alexion. Kevin H. M. Kuo: Grants/Research Support – National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health (NIH): 1R33HL147845, Thalassemia Foundation Canada, Peter Munk Cardiac Centre, University of Toronto, Cincinnati Children’s Hospital Medical Center, Canadian Hematology Society, Pfizer; Consultancy – Agios, Alexion, Apellis, Aruvant, Bluebirdbio, Celgene, Novartis, Pfizer; Chair of Data and Safety Monitoring Board (DSMB): Bioverativ/Sanofi; Scientific collaboration: Abfero, Phoenicia Biosciences. George A. Tomlinson, Richard Ward, Patricia Palcu and Nadine Shehata declare no competing financial interests.
Author contributions
A. Kinga Malinowski and Kevin H. M. Kuo share first authorship. A. Kinga Malinowski contributed to study concept design, protocol development, data collection, data interpretation, drafting of manuscript, manuscript revision and approval of final version; Kevin H. M. Kuo contributed to study concept design, protocol development, data interpretation, drafting of manuscript, manuscript revision and approval of final version. George A. Tomlinson analysed the data and contributed to its interpretation, drafting of the manuscript, manuscript revision and approval of final version; Patricia Palcu contributed to data collection, manuscript revision and approval of final version. Richard Ward contributed to data interpretation, manuscript revision and approval of final version; Nadine Shehata contributed to study concept design, data interpretation, manuscript revision and approval of final version.