Revisiting biochemistry: severe euglycemic ketoacidosis in the setting of IDH-inhibitor therapy for AML
Enasidenib is a selective inhibitor of mutated isocitrate dehydrogenase (IDH). We describe a novel presentation of life-threating euglycemic ketoacidosis, resulting from metabolite accumulation associated with the use of enasidenib.
A 70-year-old gentleman presented to the hospital with dyspnoea, fatigue and nausea for 2 days. 6 months prior, he was diagnosed with therapy-induced, CD33-positive, normal karytoype, nucleophosmin (NPM) 1 and IDH2 (R140Q) mutated acute myeloid leukemia (AML). His AML was treated initially with decitabine and venetoclax. Persistent AML (10·8% myeloblasts) was detected at the end of his first two cycles of venetoclax doublet therapy. He then underwent salvage induction chemotherapy with cladribine, cytarabine, G-CSF, mitoxantrone (CLAG-M) plus lintuzumab and achieved complete remission. His course was complicated by Serratia rubidaea bacteremia, and so enasidenib, an oral allosteric inhibitor of mutated IDH2, was initiated as consolidation and maintenance therapy, in lieu of a cytarabine-based regimen.
At the time of admission, the patient was noted to be hypothermic (36·5°C), tachypnoeic and hyperpneic. Initial laboratory results included bicarbonate 11 mmol/l, anion gap 32, glucose 133 mg/dl and creatinine 1·85 mg/dl. His liver enzymes were within normal limits and acetaminophen and salicylate levels were undetectable. Arterial blood gas demonstrated respiratory alkalosis with mixed anion gap metabolic acidosis and alkalosis with a pH of 7·44, PaO2 115, CO2 9, and an HCO3 8. His lactate was 1·1 mg/dl and acetone and beta-hydroxybutyrate levels were elevated at 48 mg/dl and 7·41 mmol/l respectively. His measured serum osmolality was 331 mOsm/kg compared to expected osmolality of 294 mOsm/kg, representing a concomitant osmolar gap. As a result of worsening respiratory status and severe acidosis, the patient was admitted to the intensive care unit and received intravenous sodium bicarbonate. Despite this treatment, he experienced persistent metabolic acidosis, which required haemodialysis. Testing for alternate causes of anion gap metabolic acidosis, hyperosmolarity and ketosis, including volatile alcohol and organic acids, was negative. We, thus, concluded enasidenib was the likely cause of his metabolic imbalances. A subsequent outpatient challenge with a reduced dose of enasidenib led to a similar, albeit milder, presentation, which worsened with a subsequent dosage increase, further substantiating this conclusion (Table 1).
| Diagnostic test | Value (normal) |
|---|---|
| Serum beta-hydroxybutyrate | 7·41 (0–0·30) mmol/l |
| Serum acetone | 48 (<10) mg/dl |
| Arterial pH | 7·44 |
| Arterial CO2 | 9 (23–27) mmol/l |
| Arterial HCO3 | 8 (21–28) mmol/l |
| Serum bicarbonate | 11 (22–29) mmol/l |
| Lactic acid | 1·1 (0·5–2·0) mmol/l |
| Urine ketones | 2+ (negative) |
We present a novel case of ketoacidosis due a targeted chemotherapeutic agent. Enasidenib is a selective inhibitor of the mutated IDH2 enzyme, which is responsible for the conversion of isocitrate to alpha-ketoglutarate in its native form. IDH is a member of a family of enzymes involved in various aspects of cellular metabolism. There are three isoforms of the IDH enzyme, which may be found in the cytosol, peroxisome and mitochondria of cells.1, 2 Laboratory models demonstrate that gain of function mutations in IDH2 drive oncogenic cellular changes; thus, enasidenib, as a selective inhibitor of this function, is used in the treatment of leukaemia.1, 2 In addition to mutations in IDH2, mutations in IDH1 have also been identified in certain cancers, including AML, and may be used for targeted treatment options.3 Mutated IDH1 or IDH2 enzymes possess a neomorphic function that results in the production of the oncometabolite 2-hydroxyglutarate (2-HG). In turn, 2-HG promotes oncogenesis through a variety of mechanisms, including histone and DNA hypermethylation and alterations in cellular metabolism.3, 4
There are several mechanisms by which IDH2 inhibition might result in ketoacidosis as it is the rate-limiting stop of the Krebs cycle. Blocking mutant IDH2 within the Krebs cycle would be likely to affect the redox ratio, balances of enzymatic products and, ultimately, regulation of mitochondrial metabolism, as seen in Fig 1. Primarily, inhibition of IDH2 in cancerous cells may lead to excess intramitochondrial accumulation of citrate. This excess, in turn, may be shuttled into the ketogenesis pathway, increasing serum acetone and beta-hydroxybutyrate levels. By inhibiting IDH2 in the Krebs cycle in healthy and malignant cells, nicotinamide adenine dinucleotide phosphate production is decreased. This change can alter the cellular redox potential and promote a cellular environment of oxidative stress.
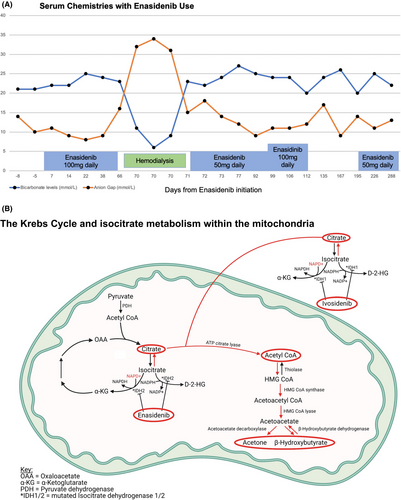
In other words, these pathways may create a biochemical milieu analogous to alcoholic ketoacidosis. Alcoholic ketoacidosis results in accumulation of acetoacetate and beta-hydroxybutyrate and an increase in the nicotinamide adenine diphosphate-hydrogen/nicotinamide adenine diphosphate+ redox ratio, thereby impairing gluconeogenesis.5 In addition to inhibition of IDH2, blockage of IDH1 with other targeted enzyme-specific chemotherapeutic agents, such as ivosidenib, may similarly contribute to this disturbance.3 Therefore, either drug has the potential to induce the metabolic disturbances manifested by this case.
Novel chemotherapeutics such as enasidenib that target metabolic pathways may cause life-threatening metabolic disturbances. Clinicians should be aware of how these agents may affect metabolic pathways in both healthy and malignant cells. As in this case, adverse effects of such medications may occur on a dose-dependent basis, which may provide opportunities for reassessment of their safe use. Ultimately, an awareness of the downstream physiological effects of medications affecting metabolic pathways can assist clinicians in predicting and detecting unintended metabolic consequences in the evaluation of cancer patients.
Author contributions
All authors contributed to the authorship and revision of this letter. Clinical care for this patient was provided by the following: Hematologic oncology (KC); Medical intensive care (MK, JR, JH, PB); Nephrology (NB).