The vasculopathic cord between pre-eclampsia and kidney function in sickle cell disease
Commentary on Boudhabhay et al, Impact of pre-eclampsia on renal outcome in sickle cell disease patients. Brit J Haematol. 2021;194:1053–1062.
In their paper Boudhabhay et al. investigate risk factors and consequences of pre-eclampsia on pregnancy outcomes and kidney function in patients with sickle cell disease (SCD).1 They report that pre-eclampsia: (i) was independently associated with higher body mass index, increased haemolysis and ≥1 vaso-occlusive crisis (VOC) requiring hospitalisation; (ii) led to poor maternal and neonatal outcomes and (iii) predicted a more rapid decline in kidney function and greater risk of developing chronic kidney disease (CKD) on longitudinal follow-up.
Pre-eclampsia affects 4–5% of pregnancies worldwide and leads to increased maternal and fetal morbidity and mortality.2 Although the exact mechanisms for developing pre-eclampsia are unclear, placental ischaemia is central in its pathogenesis. Placental ischaemia can lead to activation of hypoxia-inducible factor 1α and oxidative stress pathways, which in turn, result in increased anti-angiogenic [soluble FMS-like tyrosine kinase-1 (sFLT-1)] relative to pro-angiogenic (vascular endothelial growth factor, placental growth factor) factors and elevated levels of endothelin-1 in the circulation. In patients with SCD, the placental circulation is susceptible to impaired blood flow. Macroscopic and microscopic evaluation of the placenta from patients with SCD shows excess syncytial knots and fibrin deposition with associated villous necrosis when compared to placentas from healthy controls.3 Doppler ultrasonography studies show restricted utero-placental blood flow, which worsens during a VOC.4 The greater risk of placental ischaemia may contribute to the higher rate of maternal and fetal complications observed in SCD. In this report, Boudhabhay et al. observed pre-eclampsia in 13% (40/306) of pregnancies, similar to the incidence of pre-eclampsia (9–18%) observed in the SCD literature.5
Understanding which patients with SCD are at greatest risk of developing pre-eclampsia has been unclear. Boudhabhay et al. highlight both the degree of intravascular haemolysis and the rate of VOC as independent predictors for pre-eclampsia. Lower haemoglobin (per 10 g/l) and higher lactate dehydrogenase levels (log-transformed) at steady state and within 1-year before pregnancy predicted a 2·5-fold and 3·8-fold greater risk, respectively, of developing pre-eclampsia. A greater degree of intravascular haemolysis leads to higher circulating cell-free haemoglobin and haem concentrations, which promote vasculopathy through direct oxidative damage, upregulation of inflammatory pathways or depletion of nitric oxide.6 Haem oxygenase-1 (HMOX1) is the inducible, rate-limiting enzyme for degrading haem and serves an important role in mediating oxidative stress. Reduced HMOX1 protein has been observed in the placentas of pre-eclampsia versus normotensive non-SCD pregnancies.7 Induction of HMOX1 with cobalt protoporphyrin in a pregnant rat model of placental ischaemia attenuates hypertension.8 VOCs are a consequence of ischaemia–reperfusion injury. Having ≥1 VOC/year prior to pregnancy was associated with a 17-fold greater risk of developing pre-eclampsia. Furthermore, those patients with SCD who developed pre-eclampsia had a higher proportion with ≥1 VOC/year during their pregnancy compared those that did not have pre-eclampsia (78% vs. 48%). A higher rate of VOCs may herald a greater risk of ischaemia–reperfusion damage occurring in the placenta.
In addition to the adverse maternal and fetal pregnancy-related outcomes, there is increasing evidence that pre-eclampsia may lead to long-term cardiovascular and renal complications.2 In the general population, pre-eclampsia has been associated with a 3·7-fold greater risk of developing hypertension, a 2·2-fold greater risk of developing ischaemic heart disease and a 1·8-fold greater risk of stroke over a mean follow-up of 10–14 years.9 Pre-eclampsia may also increase the risk of kidney disease, although this association is likely bi-directional. Women who develop pre-eclampsia are at up to an eightfold greater risk of developing increased albuminuria and sixfold greater risk of developing end-stage renal disease.10, 11 Conversely, women with CKD or an acute kidney injury event prior to pregnancy are at an increased risk of developing pre-eclampsia.2 The association between pre-eclampsia and cardiovascular and renal complications may reflect either overlapping risk factors or sustained vascular damage from the pre-eclampsia.
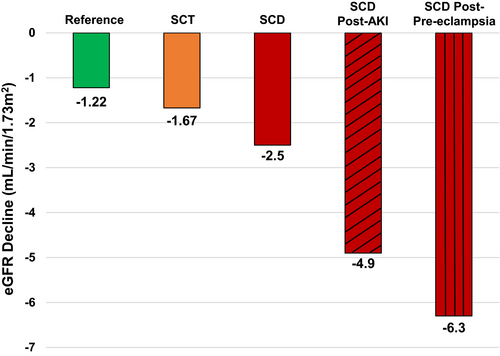
The study by Boudhabhay et al. is the first to report a link between pre-eclampsia and kidney disease in SCD. Kidney function, as determined by the estimated glomerular filtration rate (eGFR) and by urine protein-to-creatinine ratio, was similar during the first trimester of pregnancy between patients with SCD who did versus did not develop pre-eclampsia. The frequency of acute kidney injury within 48 h of delivery trended higher in those with versus without pre-eclampsia (20% vs. 9%). The eGFR was significantly lower at the time of delivery, 1 year after pregnancy, and at end of follow-up in the SCD group with versus without pre-eclampsia. Proteinuria remained significantly higher in the pre-eclampsia group at 1 year and at last follow-up compared to the group without pre-eclampsia. With a median follow-up of ˜5 years, the decline in eGFR was more rapid in patients with SCD that developed pre-eclampsia versus those that did not (−6·3 vs. −1·8 ml/min/1·73 m2 per year). Furthermore, a greater proportion of patients with SCD in the pre-eclampsia group developed CKD (58%) versus those without pre-eclampsia (9%).
Findings from this study highlight the relationship between pre-eclampsia and kidney dysfunction in SCD. Patients with SCD have a more rapid decline in eGFR compared to those with sickle cell trait or to the reference population (Fig 1).12 The rate of decline accelerates after an acute kidney injury event13 and a more rapid eGFR decline is independently associated with early mortality in adults with SCD.14 Several mechanisms implicated in SCD-related kidney disease, such as sFLT-1– and endothelin-1–mediated endothelial damage, intravascular haemolysis and impaired HMOX1 activity, and vaso-occlusive ischaemic injury overlap with the pathophysiology of pre-eclampsia.2 Pre-eclampsia may also enhance vascular response to future vessel injury, leading to greater long-term susceptibility to kidney damage.15 Future studies investigating the pathophysiological mechanisms, as well as therapies to mitigate pre-eclampsia in SCD, may help preserve long-term kidney function in this high-risk population.