Myelodysplastic syndrome patients display alterations in their immune status reflected by increased PD-L1-expressing stem cells and highly dynamic exhausted T-cell frequencies
Wiebke Moskorz and Dr. Cosmovici contributed equally as first author.
Prof. Jörg Timm and Prof. Rainer Haas contributed equally as last author.
Summary
Little data are available for the expression of immune checkpoint (IC) molecules within myelodysplastic syndrome (MDS). Here, we report increased PD-L1+CD34+CD38− and PD-L1+CD34+CD38+ stem cell frequencies within MDS patients compared to stem cell recipients in remission. Additionally, we observed exceedingly similar PD1+ and Tim-3+ T-cell frequencies between acute myeloid leukaemia (AML) and MDS samples that were elevated compared to patients in remission. Furthermore, we found highly dynamic Tim-3+ and PD1+ T-cell frequencies within serial samples of relapsing MDS with excess blasts (MDS-EB II) patients, correlating with further disease markers. These findings support the idea of a potential successful implementation of IC inhibitor treatment in suitable MDS patients.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of haematological malignancies characterized by ineffective haematopoiesis and an increased risk for progression to acute myeloid leukaemia (AML). Considering the mostly advanced age of MDS patients, alternative, less invasive treatment options, besides haematopoietic stem cell transplantations (hSCT), are urgently needed. Cancer pathogenesis is associated with exhaustion of cytotoxic lymphocytes and administration of immune checkpoint inhibitors (ICIs), that restore the function of cytotoxic cells, proved to be successful in various tumour entities (reviewed in Refs 1, 2). Importantly, ICIs have less severe side effects than conventional therapies, even in elderly patients (reviewed in Refs 3, 4). Several studies report an exhausted T-cell phenotype in AML, especially in relapsing patients (i.e. Refs 5, 6). Interestingly, only very few studies have analysed immune checkpoint (IC) molecules in MDS. Furthermore, these studies are generally based on peripheral blood (PB) cells and/or cryopreserved samples. We therefore aimed to analyse the expression of IC molecules on haematopoietic stem cells and bone marrow (BM)-infiltrating T cells that were freshly isolated via red blood cell lysis and directly subjected to flow cytometry. The study was approved by the local ethics committee (study number 3973, 3768 and 2020-1222) and informed written consent was obtained from all participants.
Seven MDS and nine AML samples were obtained at the Department of Haematology, Oncology and Clinical Immunology from the University Hospital Düsseldorf. Six hSCT recipients who remained in remission for more than six months served as controls (‘hSCT remission >6 months’). Patient characteristics are shown in Table SI.
We observed significantly increased proportions of PD-L1+CD34+ stem cells in MDS compared to hSCT recipients in remission for both the CD38− and CD38+ subset (CD38− subset: P = 0·0127 and CD38+ subset: P = 0·0336; Fig 1A, B). This is in accordance with the study from Yang and colleagues, who reported increased levels of PD-L1 mRNA transcripts in sorted CD34+ BM cells of MDS patients.7 The trend of increased PD-L1 expression could also be observed for AML patients, although not significantly. Interestingly, in MDS/AML patients the CD38+ subset showed significantly higher PD-L1+ cell proportions compared to the CD38− subset (P = 0·0135, Fig 1C), from which leukaemic stem cells (LSC) originate in both AML and MDS.8, 9 This is in line with previous reports stating that in AML and MDS PD-L1 is more commonly expressed by non-tumour cells.10 Nevertheless, these findings imply that a PD1/PD-L1 blockage could be successful not only in the treatment of AML, but also of MDS, as the LSCs comprising the CD34+CD38− subset still showed higher PD-L1 levels than in hSCT recipients in remission.
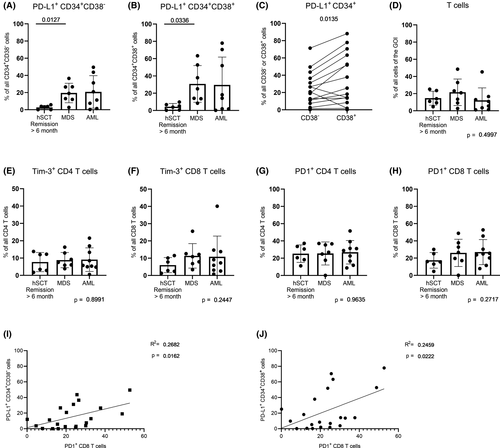
Overall T-cell as well as PD1+ and Tim-3+ CD4 and CD8 T-cell frequencies were comparable between all three groups (Fig 1D–H). In turn, several studies reported increased PD1+ and Tim-3+ CD8 T-cell frequencies in AML.5, 6 In line with these findings, we could also observe a trend towards elevated PD1+ and Tim-3+ CD8 T-cell frequencies within AML. Additionally, the corresponding T-cell frequencies within MDS were at the same levels as in AML (MDS vs AML: P = 0·9992 for both PD1 and Tim-3, Fig 1F, H). This high similarity of PD1+ CD8 T cells between MDS and AML let us assume that exhausted CD8 T cells might also be a frequent characteristic within both AML and MDS patients. This could be supported by the finding, that PD1+ CD8 T cells and PD-L1+ stem and progenitor cells in hSCT recipients showed a moderate positive correlation (CD34+CD38− subset: R2 = 0·2682, P = 0·0162 and CD34+CD38+ subset: R2 = 0·2459, P = 0·0222, Fig 1I, J).
Considering the afore-mentioned data, we questioned whether PD1+ and Tim-3+ T-cell and PD-L1+CD34+ stem cell frequencies are associated with disease progression. We therefore correlated flow cytometry data with disease markers like BM blast count, occurrence of dysplastic cells and minimal residual disease (MRD) markers in longitudinal samples of three hSCT recipients with MDS-EB II. The first patient (Fig 2A) received conditioning regimens but did not reach remission pre hSCT. Simultaneously, 11% of CD34+ progenitors were PD-L1 positive. As early as 30 days post hSCT, the patient showed a molecular relapse when the initially diagnosed trisomy 8 was detectable again. At this time point, the blast count remained below 5% but PD-L1+CD34+ progenitors were similar to the pre-hSCT value (13%). Here, the PD1+ T-cell frequencies were at their highest level in the monitoring period and decreased with administration of eight cycles of azacytidine from 55·7% to 17·3% within the CD4, and 41·0% to 19·8% within the CD8 T-cell subset. Wilms tumour protein (WT1) PB expression decreased in the same manner. Lastly, PD1+ and Tim-3+ T cells as well as WT1 expression slightly increased again. Here, PD-L1+CD34+ progenitors were hardly detectable. We therefore would recommend closer monitoring of this patient as increased Tim-3+PD1+ T cells were described in the PB of relapsed AML patients.6
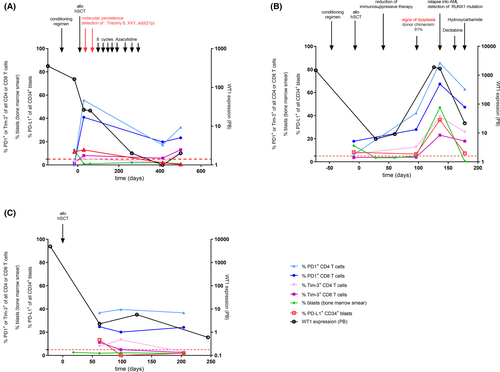
The second patient (Fig 2B) also suffered from an ongoing disease at the time point of hSCT. Already 30 days post hSCT, he showed trilineage dysplasia and a donor chimaerism drop to 72·5% (not shown). Consequently, immunosuppressive treatment was reduced to initiate a graft-versus-leukaemia effect. Thereupon, the patient reached remission with no signs of dysplasia or increased blast count. Moreover, donor chimaerism was at 93·5% at day 60 post hSCT. However, 96 days post hSCT the blast count was at 5% and dysplastic cells could be observed once again. Finally, the patient relapsed into AML 136 days post hSCT with a blast excess of 47 % and detection of the initially diagnosed RUNX1 mutation. PD1+ and Tim-3+ T cells, PD-L1+CD34+ blasts, blast count and WT1 expression tightly correlated inasmuch as they all increased simultaneously and donor chimaerism dropped to 12·8% (not shown). Administration of the first cycle of decitabine and hydroxycarbamide led to a drop in all mentioned parameters except donor chimaerism, which increased again (71·4%). Nevertheless, the proportions of Tim-3+ and PD1+ CD4 and CD8 T cells remained unexceptionally higher than the proportions at 96 days post hSCT when the first signs of dysplasia were detected before the patient relapsed into an AML.
The third patient stayed in remission over the entire monitoring period (Fig 2C). PD1+ and Tim-3+ T cells, blast count and WT1 expression were comparable between the three analysed time points. Especially PD1+ T-cell frequencies remained quite constant with frequencies fluctuating between 36·7% and 39·6% and 20·1% and 24·7% within CD4 and CD8 T cells respectively. The data presented here impressively showed that MDS-EB II disease progression included changes in the immune status. Independent of baseline levels and in contrast to the patient in remission, the relapsing patients showed highly dynamic Tim-3+ and PD1+ T-cell frequencies. Importantly, we observed these fluctuations not only within haematological but also in molecular relapse. Despite their successful administration in certain tumours, preliminary results of ICI monotherapy as single-agent treatment with nivolumab (anti-PD1) did not show an effective response in MDS patients. Yet, ICI treatment in combination with hypomethylating agents (HMAs) showed promising clinical activity in untreated higher-risk MDS and AML.11, 12 HMAs are known to increase PD-L1 expression in both AML and MDS.7, 13 Furthermore, it has been shown that tumour mutational burden (TMB) correlated with a clinical benefit from ICI therapy in several tumours, i.a. in non-small-cell lung carcinoma.14 Interestingly, PD-L1 expression seems to be thereby independent of TMB, e.g. in squamous cell lung carcinoma.15 Consequently, more criteria should be discussed for how to preselect AML/MDS patients who could benefit from ICIs in order to improve efficacy and to reduce therapy-related side effects. A therapy accompanying close monitoring of exhausted T cells could be a tool for preselection of suitable AML/MDS patients who could benefit from an ICI therapy. Furthermore, it could be used for resistance control in patients treated with HMAs. In addition, algorithms such as vi-SNE or FlowSOM should be considered in the analysis of high-dimensional flow cytometry data of serial samples, as they are able to identify residual leukaemic stem cells.16
Finally, as BM aspirations are more invasive, cost-intensive and time-consuming than blood sampling, we were interested in determining if T-cell subsets in PB and BM are comparable and could therefore be used for a therapy accompanying monitoring of exhausted T cells. As there were no matched MDS samples available, we analysed matched samples from four therapy-naïve AML patients. There were no significant differences in the proportions of exhausted CD4 and CD8 T cells as well as CD8 memory T-cell subsets (Figure S2). Consequently, we suggest that PB samples are in most cases sufficient for disease monitoring of exhausted T cells.
In summary, our data indicate that the inhibition of T cells by haematopoietic stem cells could be an underlying mechanism not only in AML, but also in MDS development. Furthermore, we could show that an intra-individual analysis of T-cell populations could be an easily applicable approach to preselect MDS and AML patients who might benefit from the use of ICIs, including resistance monitoring. Despite these encouraging findings, the present study has some limitations such as the small group size and the heterogeneous group characteristics in terms of MDS and AML subtype, treatment history, genetic aberrations and blast proportions (see Table SI). To ensure that the observations are reproducible in other subtypes and therapies, serial samples from AML/MDS patients beyond MDS-EB II who received hSCT or other types of treatment need to be analysed prospectively. As stated by Daver et al., pre-therapy PD-L1 on blasts does not correspond with a response to combination therapy of HMAs with PD-1 inhibitors within de novo and secondary AML.12 Therefore, the prospective analysis should also include patients receiving ICI treatment in order to confirm the practicality of real-time monitoring for preselecting patients for ICI treatment. Additionally, a functional analysis should be performed to determine whether the increased exhausted immunophenotype reported here directly correlates with a dysfunctional or impaired immune system. Cooperation with specialists in outpatient health care settings, where lower risk MDS is treated more frequently than in the clinics, should be therefore highly encouraged.
Acknowledgements
We thank Nicole Bombis, Stefanie Geyh, Ramona Grothmann, Irmgard Hamann, Saskia Mayer and all participating physicians for their assistance in sample collection and preparation, all patients for participating in the study and Marie-Annett Bernard for her language revisions of the manuscript. This work was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf).
Author contributions
WM performed the research. JT, RH, CC and RPC designed the research study. WM, CC and PSJ analysed the data. PSJ and RPC contributed essential materials. WM wrote the paper. All authors read and critically revised the manuscript.
Conflict of interest
All authors declare no conflicts of interest.