Outcomes and management of patients with mantle cell lymphoma after progression on brexucabtagene autoleucel therapy
Development of anti-CD19 chimeric antigen receptor T cells (CAR-T) therapies, such as brexucabtagene autoleucel (BA), is a major advance in the management of patients with relapsed refractory mantle cell lymphoma (MCL). BA is now approved by the United States Food and Drug Administration (FDA) for treating these patients. BA demonstrated an impressive 93% response rate in patients with highly refractory MCL.1 As the management and characteristics of patients with MCL after progression on BA are unknown, we describe the outcome and management of six patients with MCL after progression on BA who came off the ZUMA-2 study (ClinicalTrials.gov Identifier: NCT02601313).
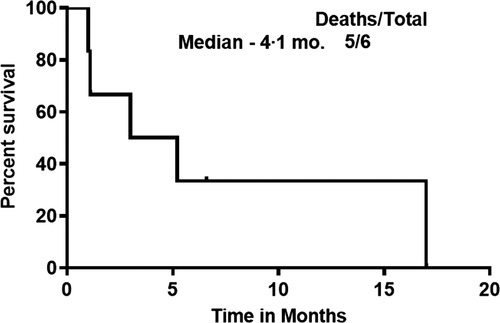
We treated 16 patients with relapsed refractory MCL with CAR-T therapies (15 on BA and one experimental CAR-T). Six of 15 patients progressed on BA. The characteristics, outcomes and clinical management of these six patients are described in Table I. Among these six patients, the median (range) time from initial diagnosis of MCL to BA infusion was 33 (20–124) months. They had a median (range) of 3 (2–4) prior therapies and all patients had progressed on prior ibrutinib. After progression, two patients had transformed to aggressive histology MCL, three did not get a biopsy and one remained as blastoid MCL. Three patients had a high Ki-67% (≥70%) after progression. Four of six patients had an extra-nodal site of relapse (two skin and soft tissues, one central nervous system and one testicular with skin involvement). We then compared the six patients who progressed with the nine patients who continued the study maintaining complete remission. These nine patients received a median (range) of 3 (2–5) lines of therapies and all had progressed on prior ibrutinib. At/before cell infusion, two had blastoid and seven classic morphology MCL, and the median (range) Ki-67% was 60 (45–100)%, in contrast, the six patients who progressed had the following features at/before cell infusion – two with blastoid and four classic morphology MCL, and the median (range) Ki-67% was 70 (20–95)%. All 15 patients received BA at the same dose of 2 × 106 T cells/kg body weight, all with similar conditioning under cohort 1 of the ZUMA-2 study. On evaluating the outcomes of the six patients who progressed, we noted that five had attained complete remission after BA and one had a partial response on BA. The median (range) response duration on BA was 6 (1·2–31·2) months. Overall, five patients had died at the time of the last follow-up. The median survival from the time of starting BA to the last follow-up was 17 months, while the median survival after progression on BA was only 4·1 months (Fig 1), with a 1-year survival rate of 0%. Patients received a median (range) of 2 (0–6) subsequent lines of therapy. The choice of subsequent therapies was based on clinical fitness of these patients, their eligibility for available clinical trials and patient choice for further therapy. Patient 1 could not get any subsequent treatment and died with progressive disease (PD), Patient 2 had stable disease and later died with PD, Patient 3 was a non-responder to subsequent treatment and Patient 4 had stable disease and later died with PD. Patient 5 is alive and under follow-up. Patient 5 had a partial response on investigational therapy. Patient 6 had a partial response on subsequent therapies, but later died with PD. As shown in Table I, a range of subsequent salvage therapies were tried including chemo-immunotherapy with/without local radiation, venetoclax, acalabrutinib, copanlisib and abemaciclib, but none of them were successful. Only patients 5 and 6 had survival beyond 6 months after progression on BA (6·6 and 17 months respectively). Compared to patients 1–4, there was no apparent difference in the cell product, processing or the characteristics of the two patients (patients 5 and 6). However, patients 5 and 6 were physically fit to receive consecutive lines of therapy and attained partial remissions, which probably was responsible for their longer survival after progression.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Survival after PD on BA, months | 1·0 | 1·1 | 3·0 | 5·2 | 6·6 | 17·0 |
| Gender (male/female) | Male | Male | Male | Male | Male | Male |
| Age, years | 52 | 55 | 69 | 62 | 68 | 67 |
| Histology type (at BA > at PD) | Classical > NA | Classical > t-Blastoid | Classical > t-Pleomorphic | t-Blastoid > NA | Classical > NA | t-Blastoid > Blastoid |
| Ki-67% (at BA > at PD) | 60 > NA | 20 > 90 | 80 > 70 | 95 > NA | 40 > NA | 80 > 80 |
| Bulky disease, yes/no | No | Yes | No | Yes | No | Yes |
| Complex karyotype, yes/no | Yes | No | No | Yes | Yes | NA |
| TP53 status | Negative | Positive | NA | Positive | Negative | NA |
| MYC status | NA | Negative | Negative | Positive | Positive | Negative |
| Number of prior lines of therapy | 3 | 3 | 3 | 3 | 2 | 4 |
| Type of prior treatments | Bort.RCAP; BR + ibrutinib; venetoclax | RCHOP; ibrutinib; auto-SCT | R-Hyper-CVAD; ibrutinib; len-rituximab-bortezomib | RHCVAD; ibrutinib; len-rituximab | Borte.-RHCVAD; ibrutinib | BR; RCHOP; ibrutinib-rituximab: R-hyper cyclophos.-dexa |
| PD on prior BTK inhibitors, yes/no | Yes | Yes | Yes | Yes | Yes | Yes |
| Prior stem cell transplant, yes/no | No | Yes | No | No | No | No |
| Time from initial diagnosis to BA therapy, months | 124·1 | 38·2 | 19·9 | 26·3 | 101·6 | 27·8 |
| Response on BA | PR | CR | CR | CR | CR | CR |
| Response duration on BA, months | 1·2 | 2·0 | 10·0 | 14·0 | 31·2 | 2·2 |
| Transformation after BA | NA | Yes | Yes | NA | NA | No |
| Site of PD after BA | LN | Soft tissues, LN | B/L testis, skin | CNS, LN, splenomegaly, perineural tissues | LN, splenomegaly | Skin and soft tissues |
| Overall survival after BA infusion to last follow-up, months | 3·3 | 3·9 | 13·9 | 20·2 | 38·7 | 20·2 |
| Number of lines of therapy after BA | 0 | 1 | 1 | 3 | 2 | 6 |
| Type of treatments after BA | NA | R-Hypercyclo. with local RT | Venetoclax | Bort.-IT ara-C+Rituximab; RT; R-Hyper-cyclophosphamide | BR; investigational targeted therapy | DR2VE; Obinutuzumab-ara-C; venetoclax; decitabine; abemaciclib; copanlisib |
| Best response to subsequent therapies | NA | SD | NR | SD | PR | PR |
| Survival status | Dead | Dead | Dead | Dead | Alive | Dead |
| Cause of death | PD | PD | PD | PD | – | PD |
| CD19 status at PD | NA | Positive | Positive | Positive | Positive | Loss |
- t-blastoid or t-pleomorphic refers to transformed blastoid or pleomorphic from classic variant mantle cell lymphoma. BA, brexucabtagene autoleucel; BR, bendamustine and rituximab; BTK, Bruton's tyrosine kinase; CAR-T, chimeric antigen receptor T cell therapy; CNS, central nervous system; CR, complete response; DR2IVE, dexamethasone, rituximab, lenalidomide and bortezomib; Len.-R, lenalidomide with rituximab; LN, lymph nodes; MINE, mesna, ifosfamide, mitoxantrone and etoposide; NA, not applicable; NR, non-responder; PD, progressive disease; PR, partial response; R-DHAP, rituximab with dexamethasone, high-dose ara-C (cisplatin); Rituximab with Hyper CVAD (cyclophosphamide, doxorubicin, vincristine) alternating with methotrexate-ara-C; RT, radiation therapy; SD, stable disease.
Fortunately, to date, we know that the numbers of patients with MCL who progress on BA are small; however, a longer follow-up of the ongoing BA studies in MCL would reveal the pattern of relapses. Our present report demonstrates that the management of MCL after progression on BA is challenging and this is an area of urgent clinical need. Although BA is a milestone advance for patients with MCL, our present report shows that patients failing BA have dismal outcomes. In addition, it is known that patients with high-risk MCL2 [aggressive histology MCL,3 high Ki-67%, tumour protein p53 (TP53) mutated4 and complex karyotype5] do not respond well to currently available therapies.
An understanding of the potential mechanisms of resistance to triple resistant MCL [Bruton's tyrosine kinase (BTK) inhibitors, venetoclax and BA] is important. Loss of CD19 antigen, CD19 splice variants, mutations in CD19, defective manufacturing of T cells, insufficient T cell expansion, aberrant cytokine milieu or functional status of CD4/CD8, upregulation of negative regulatory receptors, impact of the tumour microenvironment on T cell expansion, clonal and sub-clonal mutations and/or impaired death receptor signalling. It is unclear as to which of these mechanisms of resistance would exist in MCL and this remains to be investigated. In our present series, loss of CD19 after progression was observed in only one patient and in another patient it was unknown, but four patients retained CD19 after progression on BA. One of the caveats of our present study is lack of genomic profiling data in pre- and post-progression biopsies. It is further possible that the B-cell receptor pathway aberrations, epigenetic perturbations and derangement in metabolic reprogramming (oxidative phosphorylation), as shown by our previous reports on ibrutinib resistance6, 7 and venetoclax resistance8, play a role in mediating resistance to BA.
We further propose that clinical trials with newer agents should be considered for patients with MCL failing BA. It is possible that a combination of BTK inhibitors with BA may influence the expansion of CAR-T cells, help attain minimal residual disease negativity and may prolong duration of remission. This area is under active investigation. In addition, combination of anti- programmed cell death protein 1 (PD-1) antibodies, lenalidomide, and venetoclax with BA remains unexplored. To further speculate, since M2 polarised macrophages (CD163+), which are tumour-associated macrophages, play an important role in promoting MCL cell survival via colony-stimulating factor 1 (CSF-1),9 therefore, blockage of the CSF-1 receptor by pexidartinib or via anti-granulocyte-macrophage (GM)-CSF monoclonal antibody lenzilumab with BA might be another potential therapeutic strategy in MCL progressing after BA.
Overall, we believe that although the advent of BA has ushered in a new era in the therapy of MCL, at the same time, this has posed several important questions, which need to be addressed in the forthcoming studies. The mechanisms of resistance to BA and clonal and sub-clonal evolution mediating resistance is under investigation by our group. It is possible that newer agents and newer combination clinical trials may help in salvaging patients who progress on BA. We believe that using a combination of BTK inhibitors with BA at earlier time points during the course of MCL may delay the development of BA resistance.
Conflict of Interest
None of the authors declare any competing financial interests related to the patients described in this manuscript. Otherwise, no other competing conflicts are reported by other authors except for Michael L. Wang – stock or other ownership in MoreHealth; honoraria from Pharmacyclics, Janssen, AstraZeneca/Acerta Pharma, Targeted Oncology, and OMI; consultancy or advisory role for Pharmacyclics, Celgene, Janssen, AstraZeneca/Acerta Pharma, MoreHealth, Loxo Oncology, Kite, a Gilead Company, and Pulse Biosciences; research funding from Pharmacyclics, Janssen, AstraZeneca/Acerta Pharma, BioInvent, Novartis, Kite, a Gilead Company, Juno, Celgene, Loxo Oncology, and VelosBio; expert testimony for AstraZeneca/Acerta Pharma; and travel support from Janssen, Pharmacyclics, Celgene, Targeted Oncology, and OMI.
Author Contributions
Preetesh Jain and Michael L. Wang designed, collected, managed patients and wrote the manuscript. Preetesh Jain, Lucy Navsaria and Omar Moghrabi collected the data and wrote the paper. All authors provided contributions to the analysis and the interpretation of data for the work, helped in drafting the work and revised it critically for important intellectual content and gave final approval of the version to be published and were in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.