Obinutuzumab in the treatment of autoimmune haemolytic anaemia and immune thrombocytopenia in patients with chronic lymphocytic leukaemia/small lymphocytic lymphoma
Obinutuzumab is a type II, glycoengineered, humanized anti-CD20 monoclonal antibody, with enhanced direct cell death and antibody-dependent cell-mediated cytotoxicity.1 Obinutuzumab is FDA-approved for chronic lymphocytic leukaemia (CLL) in combination with chlorambucil, ibrutinib or venetoclax as a first-line treatment.2-4 Similar to rituximab, obinutuzumab induces B-cell depletion and reduced antibody production, granting it the potential to effectively treat antibody-mediated autoimmune phenomena. We report here a retrospective analysis of eight patients with CLL/small lymphocytic lymphoma (SLL) who were treated with single-agent obinutuzumab for autoimmune haemolytic anaemia (AIHA) or immune thrombocytopenia (ITP), from September 2018 to March 2020. Obinutuzumab was given in a similar manner as reported in the CLL11 trial study but without chlorambucil.2 (For more details on inclusion criteria, response evaluation and statistical methods see Data S1). The patients had no International Workshop on Chronic Lymphocytic Leukemia (IWCLL) 2018 indication for treatment aside from AIHA or ITP poorly responsive to corticosteroids. Baseline demographic and disease characteristics are listed in Table I. Four patients were treated for IgG–warm antibody AIHA (all steroid-dependent) and four were treated for ITP (one primary-refractory to multiple lines of therapy including rituximab and splenectomy, one steroid-dependent and two relapsing after discontinuation of corticosteroids). Five patients were able to discontinue corticosteroids at some point during the disease course, prior to treatment with obinutuzumab, with a median duration of response to corticosteroids of 4·1 months (range, 0·6–19·7; Table).
| Patient # | Sex | AIHA/ITP | FISH | IGHV | Response to steroids | Prev. lines (no.) | Prev. treatments |
DOLR before obinutu (months) |
Min Hb (g/l) or plts (109/l),during 30 days Before obinutu |
Hb (g/l) or plts (109/l), D1-obinutu |
Lymphocyte count (109/l), D1-obinutu |
Steroids dosage (mg), D1-obinutu |
DOR (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | AIHA | nd | UM | Dependent | 4 |
Pred Rituxi |
19·7 | 114 | 151 | 10·6 | 40·00 | 17·9+ |
| 2 | M | AIHA | del17p | UM | Dependent | 1 | Pred | Without remission | 64 | 94 | 136·9 | 60·00 | 14·4+ |
| 3 | F | AIHA | 12+ | UM | Dependent | 1 | Pred | Without remission | 80 | 96 | 189·7 | 60·00 | 13·9+ |
| 4 | M | AIHA | nd | UM | Dependent | 1 | Pred | 4·1 | 95 | 91 | 6·5 | 25·00 | 10·4+ |
| 5 | M | ITP | del11q | UM | Refractory | 8 |
Pred Splenectomy Azathioprine Danazol IVIG Rituxi Eltrombopag Romiplostim |
without remission | 25 | 25 | 3·9 | 25·0 | 18·9 |
| 6 | F | ITP | del17p | UM | Dependent | 2 |
Pred IVIG |
0·6 (18 days) | 9 | 269 | 118·4 | 20·0 | 19·5+ |
| 7 | F | ITP | del13q | UM | Relapsing-responsive | 2 | Pred | 4·4 | 62 | 159 | 22·2 | 20·0 | 4·6+ |
| 8 | F | ITP | nd | UM | Relapsing-responsive | 5 | Pred | 3·2 | 19 | 147 | 25·3 | 10·0 | 4·6 |
- AIHA, autoimmune haemolytic anaemia; DOLR, duration of last remission; DOR, duration of response; FISH- fluorescence in-situ hybridization; Hb, haemoglobin; ITP, immune thrombocytopenia; IGHV, immunoglobulin heavy chain variable region gene mutation status; IVIG, intravenous immunoglobulin; Min, minimum; no., number; nd, no data; Obinutu, obinutuzumab; Plts, platelets; Pred, prednisone; Prev., previous.
All patients with AIHA were treated with corticosteroids before starting obinutuzumab and the median number of prior therapies was 1 (range, 1–4). The mean value of lowest haemoglobin level recorded for each patient during the 30 days prior to initiation of obinutuzumab treatment was 87.5 ± 21 g/l and the mean haemoglobin level recorded on the first day of obinutuzumab cycle 1 was 108 ± 29 g/l (Fig 1A).
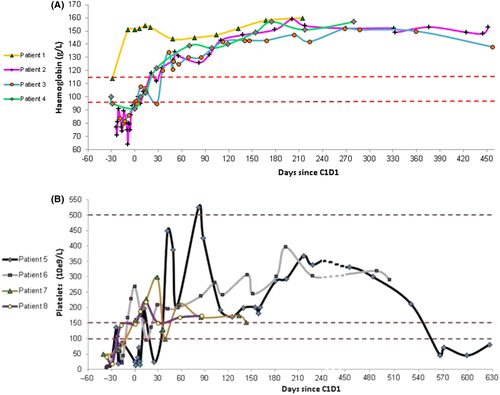
Among patients with ITP, three were treated solely with corticosteroids at the period prior to starting obinutuzumab and one patient was treated with a combination of corticosteroids and romiplostim. The lowest median platelet count, recorded for each patient, during 30 days prior to the first administration of obinutuzumab was 19 × 109/l (range, 9–62 × 109) and, the median platelet count on the first day of obinutuzumab treatment was 153 × 109/l (range, 25–269 × 109/l) Fig 1B. The median number of prior therapies for ITP was 3·5 (range, 2–9).
At the time of obinutuzumab initiation, the median prednisone dose for all patients was 27·5 mg (range, 10–60). The median cumulative dose of obinutuzumab given, for the entire cohort was 8,000 mg (range, 6,000 – 8,000). The two patients who did not receive the full scheduled dose of treatment discontinued obinutuzumab, due to the COVID19 pandemic.
In the AIHA cohort, complete response (CR) of the autoimmune cytopenia (AIC) was observed in all four patients (100%) and, in these cases, enabling discontinuation of corticosteroids. The median time to achieve CR was 54·7 days. During a median follow-up of 15·1 months (range, 11·3–17·9), all patients had maintained response Fig 1A.
Among the patients with ITP, CR of the AIC was achieved in all four patients (100%), including a patient who had previously failed multiple lines of treatments, including rituximab and splenectomy. In patient #5, who was still thrombocytopenic on the day of starting obinutuzumab, the time to response was 11 days and the time to CR of the AIC was 42 days. Therapy with corticosteroids was discontinued in all four cases. During a median observation period of 11·7 (4·6–19·4) months, one patient with ITP relapsed after being 18·9 months in remission; he was retreated with obinutuzumab combined with venetoclax and achieved a second CR (Fig 1B).
In the entire cohort, the median time for discontinuation of steroids was 1·3 months (range, 0·23–5·57) and patient #5 discontinued romiplostim after 1·8 months.
The overall clinical response rate for CLL was 87·5% (7/8), including CRs in four (50%) patients, partial responses (PRs) in three (37·5%) and one with stable disease.
None of the patients had neutropenic fever episodes, hospitalization or dose reductions due to toxicities. Infusion-related reactions (IRR) grade 1–2 were noted in two patients (25%). During the period of treatment, one patient (12·5%) developed two episodes of grade 3 and 4 neutropenia that was treated with granulocyte-colony-stimulating factor and eventually resolved.
Taken together, we report here, for the first time, the efficacy of obinutuzumab for treatment of AIHA and ITP in patients with CLL/SLL. Obinutuzumab was highly efficacious, achieving a CR rate for the AIC of 100%. During a median follow-up period of 15 months, relapse occurred only in a single patient with ITP (after 18·9 months). Responses were observed early and CRs were achieved in less than eight weeks. The patients in our cohort failed to obtain adequate and sustained responses to previous corticosteroid therapy. However, following treatment with obinutuzumab, corticosteroids could be discontinued in all cases. Among our patients, the results achieved with obinutuzumab were especially impressive in a patient with ITP, resistant to multiple therapies, including splenectomy and rituximab, who rapidly responded to obinutuzumab.
In general, treatment with rituximab for relapsed/refractory warm antibody AIHA achieves an overall response (OR) rate and CR rate of approximately 70–80% and 40%, respectively.5, 6 The median time to response is 4–6 weeks and the median duration of response is about one year.6-8 In adult patients with ITP, treatment with rituximab obtains responses and CRs in about 60% and 40% of cases, respectively.9-12 However, long-term follow-up data showed that only 20–30% of patients maintained the remission.12
In summary, despite the limitations of this study, being a retrospective study, with a small number of patients and a relatively short median follow-up, our data clearly shows that obinutuzumab is a highly effective treatment for AIHA and ITP in patients with CLL/SLL. A prospective randomized trial comparing the efficacy of obinutuzumab and rituximab in the treatment of AIHA/ITP in patients with CLL/SLL, as well as the optimal duration of treatment (e.g. short-term obinutuzumab), is warranted.
Author contribution
YH initiated and supervised the study and wrote the manuscript; TK, YB, DV, IA, RR and NB collected data and critically reviewed the manuscript; SL analyzed the data; CP wrote the manuscript.
Conflict of interest
YH — honoraria from Janssen, AbbVie, AstraZeneca, Sanofi, Medison and Roche.




