Early relapse after high-dose melphalan autologous stem cell transplant predicts inferior survival and is associated with high disease burden and genetically high-risk disease in multiple myeloma
Summary
Predicting patient outcome in multiple myeloma remains challenging despite the availability of standard prognostic biomarkers. We investigated outcome for patients relapsing early from intensive therapy on NCRI Myeloma XI. Relapse within 12 months of autologous stem cell transplant was associated with markedly worse median progression-free survival 2 (PFS2) of 18 months and overall survival (OS) of 26 months, compared to median PFS2 of 85 months and OS of 91 months for later relapsing patients despite equal access to and use of subsequent therapies, highlighting the urgent need for improved outcome prediction and early intervention strategies for myeloma patients.
Introduction
High-dose melphalan (HDM)-conditioned transplantation in myeloma has become a standard of care in first remission justifiable by prolonged progression-free survival (PFS) and overall survival (OS) compared to chemotherapy alone.1 Factors known to be associated with longer PFS following autologous stem cell transplantation (ASCT) include achievement of a deep response [very good partial response (VGPR)/complete response (CR)]2 and lenalidomide maintenance.3, 4 Nevertheless, not all patients benefit from standard treatments and the ability to predict which patients have disease that progresses early will improve outcomes. We used data from the Myeloma XI trial to explore this.
Patients and methods
Myeloma XI compared immunomodulatory drug (IMiD)-based triplet induction with thalidomide or lenalidomide combined with cyclophosphamide and dexamethasone. Patients who did not achieve at least a VGPR were randomised to proteasome inhibitor-based therapy (bortezomib, cyclophosphamide and dexamethasone) or no further therapy prior to ASCT. Lenalidomide-based maintenance treatment was also compared to no further therapy following ASCT. The results of these randomisations have been published or are in submission.3, 4
The objectives of this analysis were to compare the baseline characteristics, cytogenetics, treatment, response and outcome in participants who relapsed within 12 months post HDM–ASCT to those who did not using the two-sample t-test for continuous variables and the chi square test for categorical variables. In addition, the aim was to assess OS and PFS2 based on time to first relapse post HDM–ASCT. PFS2 was defined as the time from HDM administration to the time of second documented disease progression (or the start of the next line anti-myeloma treatment) or death from any cause, whichever occurs first. OS was defined as the time from HDM administration to death from any cause. Individuals were censored at the last date they were known to be alive and second progression-free for PFS2, and alive for OS. The survival function for PFS2 and OS was estimated using the Kaplan–Meier method. We compared PFS2 and OS using ratios of incidence rates per person-month measured in follow-up after first relapse. Incidence rates and rate ratio were assessed using a normal approximation to the Poisson distribution.
Two groups of patients were defined by the time of their first relapse. The early relapse (ER) group comprised participants with progressive disease or a myeloma-related death within 12 months post HDM administration. Participants who died within 12 months post HDM administration without evidence of progressive disease were not included in this group and were censored at the time of death. The non-early relapse (nER) group comprised of all other participants in the analysis population who did not have progressive disease or a myeloma-related death within 12 months post HDM administration. Cytogenetic risk was analysed in a subset of patients. Risk was defined as5, 6 the presence of any one [high risk (HiR)] or more than one [ultra-high risk (UHiR)] of the following lesions: del(17p), gain(1q), t(4,14), t(14;16) or t(14;20).
Results
Patients and characteristics
Of the 1 349 patients who completed ASCT within the trial, 174 (12·9%) were in the ER group and 1 175 (87·1%) were in the nER group. Within the nER group, 649 participants (55·2%) had a progression or death event, and 526 participants (44·8%) were alive and progression-free at the time of analysis. Comparison of the baseline characteristics of participants at initial randomisation demonstrates that haemoglobin concentration, platelets, plasma cell percentage in the bone marrow, β2 microglobulin, calcium levels and International Staging System (ISS) stage significantly differed between the relapse groups (Table I). All other characteristics examined did not (Table SI).
| Clinical characteristics | ER (n = 174) | nER (n = 1175) | Total (n = 1349) | P |
|---|---|---|---|---|
| Haemoglobin (g/l) | ||||
| Mean (SD) | 104·4 (19·33) | 111·9 (20·04) | 110·9 (20·09) | <0·0001 |
| Median (range) | 104·5 (60·0, 160·0) | 111·0 (33·0, 174·0) | 110·0 (33·0, 174·0) | |
| Platelets (× 109/l) | ||||
| Mean (SD) | 226·5 (91·19) | 257·6 (101·62) | 253·6 (100·84) | 0·0001 |
| Median (range) | 222·5 (34·0, 622·0) | 245·0 (2·0, 1112·0) | 242·0 (2·0, 1112·0) | |
| Plasma cells (%) | ||||
| Mean (SD) | 43·5 (26·61) | 34·6 (25·05) | 35·7 (25·42) | <0·0001 |
| Median (range) | 40·0 (1·0, 142·0) | 29·0 (0·0, 100·0) | 30·0 (0·0, 142·0) | |
| Missing | 12 | 88 | 100 | |
| β2 microglobulin (mg/l) | ||||
| Mean (SD) | 5·4 (4·44) | 4·5 (4·50) | 4·6 (4·50) | 0·0002 |
| Median (range) | 4·1 (1·3, 29·0) | 3·3 (1·0, 81·0) | 3·4 (1·0, 81·0) | |
| Missing | 15 | 86 | 101 | |
| Calcium (mmol/l) | ||||
| Mean (SD) | 2·5 (0·33) | 2·4 (0·25) | 2·4 (0·26) | 0·0382 |
| Median (range) | 2·5 (1·6, 3·8) | 2·4 (1·3, 4·6) | 2·4 (1·3, 4·6) | |
| Missing | 0 | 5 | 5 | |
| ISS | ||||
| Stage I | 39 (22·4%) | 409 (34·8%) | 448 (33·2%) | 0·0029 |
| Stage II | 73 (42·0%) | 442 (37·6%) | 515 (38·2%) | |
| Stage III | 47 (27·0%) | 236 (20·1%) | 283 (21·0%) | |
| Missing | 15 (8·6%) | 88 (7·5%) | 103 (7·6%) | |
| Cytogenetics | ER (n = 78) | nER (n = 487) | Total (n = 565) | P |
|---|---|---|---|---|
| gain(1q) | ||||
| Present | 39 (50·0%) | 139 (28·5%) | 178 (31·5%) | 0·0002 |
| Absent | 39 (50·0%) | 348 (71·5%) | 387 (68·5%) | |
| t(4,14) | ||||
| Present | 31 (39·7%) | 51 (10·5%) | 82 (14·5%) | <0·0001 |
| Absent | 47 (60·3%) | 436 (89·5%) | 483 (85·5%) | |
| t(14,16) | ||||
| Present | 3 (3·8%) | 11 (2·3%) | 14 (2·5%) | 0·4242 |
| Absent | 75 (96·2%) | 476 (97·7%) | 551 (97·5%) | |
| del(17p) | ||||
| Present | 15 (19·2%) | 30 (6·2%) | 45 (8·0%) | 0·0001 |
| Absent | 63 (80·8%) | 457 (93·8%) | 520 (92·0%) | |
| t(4,14) and/or del(17p) | ||||
| Present | 42 (53·8%) | 78 (16·0%) | 120 (21·2%) | <0·0001 |
| Absent | 36 (46·2%) | 409 (84·0%) | 445 (78·8%) | |
| t(14,16) and/or t(14,20) | ||||
| Present | 3 (3·8%) | 14 (2·9%) | 17 (3·0%) | 0·7174 |
| Absent | 75 (96·2%) | 473 (97·1%) | 548 (97·0%) | |
| Cytogenetic risk | ||||
| SR | 22 (28·2%) | 298 (61·2%) | 320 (56·6%) | <0·0001 |
| HiR | 28 (35·9%) | 146 (30·0%) | 174 (30·8%) | |
| UHiR | 28 (35·9%) | 43 (8·8%) | 71 (12·6%) | |
- ER, early relapse; nER, non-early relapse; ISS, International Staging System; SR, standard risk; HiR, high risk; UHiR, ultra-high risk.
More patients were classified as high risk (HiR) or ultra-high risk (UHiR) in the ER group (Table I). Gain(1q), t(4,14), del(17p), ‘t(4,14) and/or del(17p)’ and cytogenetic risk significantly differed between the relapse groups.
The number of patients with UHiR genetics was almost fourfold higher in the ER group compared to the nER group. In addition, each of the high-risk lesions del(17p) and t(4;14) were present in three times more patients with ER compared to nER. The same was not true for t(14;16) and t(14;20) although absolute numbers of patients with these lesions was low.
Comparison of trial treatment received
There were no differences in the randomised trial treatment during the induction and consolidation phases according to relapse group; however, the randomised maintenance treatment did differ between groups (Table SII).
Patients in the ER group were less likely to have received lenalidomide-based maintenance compared to those in the nER group (35·7% vs. 46·5%; P = 0·0005). However, 41/115 (35·7%) of patients who relapsed within 12 months were on maintenance treatment, suggesting that continuous therapy does not prevent early disease progression in all.
Depth of response
Responses at the end of induction treatment were deeper in the nER group, with 72·5% of the nER group with VGPR or better compared to 64·4% in the ER group (Table SIII). A similar pattern was seen at 100 days post melphalan where 24 participants (13·8%) in the ER group had progressive disease (PD) and thus obtained no benefit from the procedure.
Subsequent treatment and survival
Overall, 80·8% of ER patients and 76·5% of nER patients received second-line treatment post progression, 49·1% of ER patients and 40·8% of nER received third-line treatment and 25·1% of ER and 19·6% of nER patients received subsequent lines of therapy at the time of analysis. Bortezomib combinations were the most common second-line treatment in both groups, given in more than two thirds of cases and lenalidomide-based regimens the most common third-line treatment given in approximately half of cases.
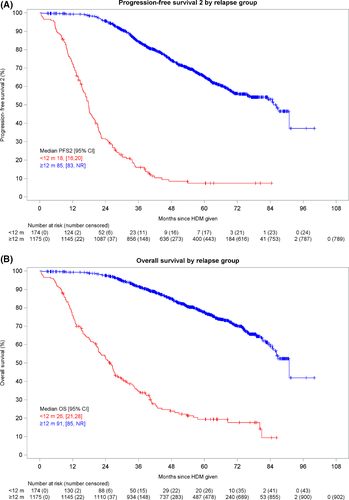
Early relapse was associated with short PFS2 (Fig 1A), with a median of 18 months (95% CI: 16, 20) for the ER group as compared to 85 months for the nER group [95% CI: 83; Not Estimable (NE)]. After first relapse there were 150 PFS2 events in 2376 person-months for the ER group [incidence rate per 12 person-months (IR per 12 pm): 0·76; 95% CI: 0·65, 0·89] and 386 PFS2 events in 38 516 person-months for the nER group (IR per 12 pm: 0·12; 95% CI: 0·11, 0·13], risk ratio (RR) 6·30, 95% CI: 5·22, 7·61; score test P < 0·001). Similarly, early relapse was associated with short OS (Fig 1B), with a median of 26 months (95% CI: 21, 28) for the ER group and 91 months (95% CI: 85, NE) for the nER group. After first relapse there were 131 deaths in 3 681 person-months for the ER group (IR per 12 pm: 0·43; 95% CI: 0·36, 0·51) and 273 deaths in 42 521 person-months for the nER group (IR per 12pm: 0·08; 95% CI: 0·07, 0·09; RR 5·54, 95% CI: 4·50, 6·83; score test P < 0·001). Almost three quarters of those in the ER group had died within three years of trial entry.
Discussion
This is a focussed analysis of outcomes for patients treated intensively who relapse within 12 months of ASCT in the Myeloma XI trial and confirms that their prognosis is poor compared to patients who relapse thereafter. It was not possible to salvage the majority of early relapsing patients, despite treatment with multi-novel-agent therapy, suggesting that the underlying factors for early relapse are predominantly disease-inherent, not treatment-specific, warranting the search for novel prognostic tools. This analysis confirms that we are unable to identify all high-risk patients at diagnosis using current staging systems. Certain clinical characteristics were shown to be more common in patients who relapse early, for example lower haemoglobin concentration and platelet count together with heavier bone marrow plasma cell infiltration, in keeping with higher disease burden and less functional reserve. However, there was no significant difference in surrogate markers of disease burden paraprotein, serum-free light chain, serum creatinine or lactate dehydrogenase (LDH) level.
We observed that 35·9% of patients in the early relapse group had one high-risk lesion and the same number had more than one (UHiR) which is more than treble the rate seen in nER patients. Availability of detailed genetic information demonstrates that approximately 70% of early relapse patients carry a high-risk genetic lesion. Although more patients in the short PFS group had HiR or UHiR genetics and ISS 3 disease, early progression was also seen in patients lacking any of the risk markers analysed and understanding this phenomenon will be essential for improved prognostic tools.7 It is the topic of ongoing research to identify additional predictive markers for early relapse. Whole-body diffusion-weighted magnetic resonance imaging (MRI) or circulating tumour DNA assessment to extrapolate spatial heterogeneity that is cryptic to bone marrow biopsy-based assessment as performed here are emerging as promising strategies.8, 9
In some cases, early relapse in Myeloma XI was seen following deep serological responses to induction therapy, ASCT and during lenalidomide-based maintenance treatment. This observation challenges the notion of a one-size-fits-all approach to treatment and future trials will focus on risk-adapted maintenance therapy such as UK OPTIMUM MUKnine (ISRCTN16847817). The ability to predict prognosis on the basis of diagnostic clinical, laboratory and genetic factors is a key goal of personalised therapy in myeloma, but the penetrance of standard genetic abnormalities is heterogeneous and likely combines with additional patient, treatment-acquired and tumour-specific factors to generate an individual’s level of risk.
In conclusion, anaemia, low platelet count, heavy plasma cell infiltration, advanced ISS stage and high-risk genetic markers are clinical candidates as prognostic markers for early relapse in MXI. Early relapse was independent of all other clinical characteristics and type of induction treatment. Patients who relapse early are hard to salvage and more likely to die prematurely from their disease. Further characterisation and prioritisation of this group of patients for new therapeutic approaches remains an important area of development with the aim of improving the outlook for patients with phenotypically high-risk myeloma.
Acknowledgements
We thank all the patients at centres throughout the United Kingdom whose willingness to participate made this study possible. We are grateful to the UK National Cancer Research Institute Haematological Oncology subgroup, UK Myeloma Research Alliance, and to all principal investigators, sub-investigators, and local centre staff for their dedication and commitment to recruiting patients to the study. We thank the members of the Myeloma XI Trial Steering Committee and Data Monitoring and Ethics Committee. The support of the Clinical Trials Research Unit at the University of Leeds was essential to the successful running of the study; we thank all their staff who have contributed, past and present. Central laboratory analysis was performed at the Institute of Immunology and Immunotherapy, the University of Birmingham; the Institute of Cancer Research, London; and the Haematological Malignancy Diagnostic Service, St James’s University Hospital, Leeds. We are very grateful to the laboratory teams for their contribution to the study. We also acknowledge support from the National Institute of Health Biomedical Research Centre at the Royal Marsden Hospital and the Institute of Cancer Research.
Funding
Financial support for the Myeloma XI trial was obtained from Cancer Research UK [C1298/A10410]. Unrestricted educational grants from Celgene Corporation and Merck Sharp and Dohme, as well as funding from Myeloma UK supported the trial coordination and the laboratory studies. MK was supported by a Jacquelin Forbes-Nixon Fellowship.




