Progressive increase in FIX level in males with haemophilia B Leyden and c.35G > A mutation in early childhood not related to androgen effect
Haemophilia B is a congenital X-linked recessive bleeding disorder that occurs as a result of mutations at the F9 gene located at Xq27.1 It affects 1 in 30 000 males.2 The severity of haemophilia B correlates with the residual circulating level of plasma FIX:C and is determined by the specific genetic lesion. There are 1 094 unique variants giving rise to haemophilia B as per the Factor IX Gene Variant Database, accessed January 2020. Haemophilia B Leyden was first recognised in 1970 with the observation of a progressive rise in FIX:C level from the time of puberty at a rate of about 5% per year to reach a lower normal level.3-5 There are more than 20 genetic mutations that are associated with the haemophilia B Leyden phenotype. These mutations cluster at three particular regions within the proximal promoter region of the F9 gene and include mutations in nucleotides c.−34 and c.−35 (−5 and −6 legacy numbering) in almost half the cases, mutations around nucleotide c.−49 (−20) and mutations around nucleotide c.−19 (+10).6
The mechanisms for post-pubertal increase of FIX in haemophilia B Leyden are controversial with most in vitro and in vivo studies suggesting a direct role of androgen receptors.7-9 Androgenic steroids were used for prophylactic treatment in patients with haemophilia B Leyden.10 In a study using transgenic mice recapitulating the phenotype of haemophilia B Leyden, human FIX expression was reproducibly nullified by hypophysectomy, but nearly fully restored by administration of growth hormone (GH), providing strong evidence that GH is directly responsible for puberty onset recovery of FIX production. On the contrary, FIX:C levels were restored to only 10% of pre-hypophysectomy levels by administration of dihydrotestosterone or oestradiol.11 A report of two symptomatic female carriers of haemophilia B Leyden showed age-related normalisation of their plasma FIX:C level with no clinical features of androgen excess.12 In 1993 a case of haemophilia B associated with a c.−35G>A mutation in the promoter gene was reported in a baby who presented at the age of four weeks with suspected intracranial bleeding. His FIX:C level was <1% at diagnosis and it increased early in childhood with FIX:C of 20% at four months and 22–25% at ten years of age with no further bleeding symptoms.13
Patients and methods
Haemophilia B is twice as common in Ireland compared to other countries (one per 12 500 males). Mutation at c.−35G>A (legacy number g.−6 G>A) in the 5' UTR is the second most common mutation in mild haemophilia B in Ireland.14 We studied haemophilia B Leyden in males attending Children's Health Ireland, to evaluate the timing of increase in their plasma FIX:C level. We measured the level of FIX:C by automated one-stage assay using the same assay and reagents in 13 male children diagnosed with haemophilia B Leyden from seven apparently unrelated Irish families. Sequence analysis was performed by standard dideoxysequencing of F9 exon 1 and the upstream promoter region.14
Results
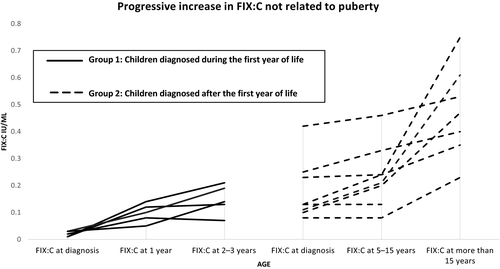
The hemizygous presence of the haemophilia B Leyden associated variant c.−35G>A was confirmed by standard dideoxysequence analysis of 13 male children (Table I). These children were divided into two groups depending on the age at diagnosis. Group One included five children diagnosed during the first year of life. Group Two comprised eight children diagnosed after one year of age.
|
Group 1: Children diagnosed with haemophilia B Leyden during the first year of life |
Group 2: Children diagnosed with haemophilia B Leyden after the first year of life |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Child | Age at diagnosis | FIX:C at diagnosis iu/ml | PT at diagnosis (NR * s) | FIX:C at 1 year iu/ml | FIX:C at 2–3 years iu/ml | Child | Age at diagnosis | FIX:C at diagnosis iu/ml | FIX:C at 5–15 years iu/ml | Last FIX:C iu/ml (Age at the time of testing) |
| 1 | 1 month | 0·02 | 13·4 (NR 9·3–14·3) | 0·08 | 0·07 | 6 | 3 years | 0·13 | 0·13 | 0·11 (5 years) |
| 2 | 9 days | <0·01 | 12·9 (NR 9·3–14·3) | 0·12 | 0·13 | 7 | 7 years | 0·23 | 0·24 | 0·35 (16 years) |
| 3 | 1 day | <0·01 | 12·9 (NR 10·1–15·9) | 0·14 | 0·21 | 8 | 9 years | 0·08 | 0·08 | 0·23 (16 years) |
| 4 | 9 months | 0·03 | 11·6 (NR 10·6–11·4) | 0·1 | 0·19 | 9 | 8 years | 0·13 | 0·24 | 0·75 (17 years) |
| 5 | 3 months | 0·03 | 11·2 (NR 9·3–14·3) | 0·05 | 0·14 | 10 | 12 years | 0·10 | 0·2 | 0·47 (15 years) |
| 11 | 5 years | 0·11 | 0·21 | 0·61 (14 years) | ||||||
| 12 | 14 years | 0·25 | 0·33 | 0·4 (18 years) | ||||||
| 13 | 11 years | 0·46 | 0·42 | 0·53 (15 years) | ||||||
- * NR, normal prothrombin time (PT) range for age.
In all five children in Group One, FIX:C level increased progressively from the severe/moderate range to the mild range before three years of age (Fig 1). All eight children in Group Two had mild FIX deficiency at diagnosis. Seven of eight children in Group Two showed an increase in FIX:C level from pre/early puberty to late puberty; there were not enough data on one child as his last FIX:C level was taken when he was five years old (Table I).
Discussion
Serial measurements of FIX:C levels in children with haemophilia B Leyden and c.−35G>A mutation showed increase in FIX plasma levels from the severe/moderate FIX range to mild range during the first three years of life independent of androgen effect (Fig 1). Children diagnosed during the neonatal period had normal prothrombin time (PT) at diagnosis, making other factors that can contribute to low FIX:C level such as low vitamin K, hepatic dysfunction or disseminated intravascular coagulation (DIC) extremely unlikely (Table I). All children diagnosed after the first year of life had mild haemophilia B with factor IX:C level that continued to increase with age with a more pronounced increase during puberty. These findings are in line with animal studies suggesting GH as the driver for surges in FIX:C level in haemophilia B Leyden. We did not measure GH levels; however, growth and development of children in this study was normal and none of the children in the study showed features suggestive of growth retardation. GH secretion is pulsatile, with consistent surges during sleep. Between normal pulses of GH secretion, serum GH levels are often low; thus, measurement of a single serum GH level is not helpful.15, 16 GH secretion is low during infancy and increases but remains stable at low levels during early childhood, until just before puberty when GH secretion surges to the highest lifetime peak.15 All cases in our cohort have an identical F9 mutation. Our findings suggest that the heterogeneity of haemophilia B Leyden genetic mutations is also associated with heterogeneity in the mechanisms through which FIX:C plasma levels increase with age.
Acknowledgements
SA performed the research and wrote the paper; MO and IR collected the laboratory and clinical data; VJ performed the genetic analysis and reviewed the paper; BN designed the study and reviewed the paper.