Lactate dehydrogenase is elevated in immune thrombocytopenia and inversely correlates with platelet count
Lactate dehydrogenase (LDH) is present in numerous cell types, including platelets, which have high LDH activity (Hule, 1966). Plasma LDH levels are routinely obtained to develop or refine a differential diagnosis. Significant elevations are seen in various malignancies, tissue necrosis, haemolytic anaemia, ineffective erythropoiesis, liver disease, nephrotic syndrome and muscular disorders (Marshall et al, 1991; Tietz, 1995). Although widely used as a marker of erythrocyte destruction, we are unaware of published data describing LDH elevation in elevated platelet turnover without concurrent erythrocyte destruction, as occurs in immune thrombocytopenia (ITP). This is diagnostically relevant because several secondary causes of ITP, differential diagnoses and complicating illnesses are associated with LDH elevation. After anecdotal recognition of LDH elevations in ITP patients with no evidence of haemolysis, we examined LDH levels in ITP patients attending our centre. We hypothesized that elevated LDH was common in ITP and that the degree of elevation inversely correlated with platelet count. Plasma LDH is routinely obtained in ITP patients in our centre given the aforementioned diagnostic associations.
ITP patients aged ≥18 years at diagnosis fulfilling American Society of Hematology ITP diagnostic criteria (Neunert et al, 2011) with one or more LDH levels obtained between 1 January 1990 and 31 December 2018 were identified. Exclusion criteria included known complicating haemolytic anaemia (Evans syndrome) or positive direct antiglobulin test (DAT) in the absence of clinical haemolysis, bilirubin elevation, liver disease, malignancy, shock, nephrotic syndrome, megaloblastic anaemia, muscular disorder, intravenous immunoglobulin treatment within 4 weeks, or treatment with dapsone or anti-RhD immunoglobulin at any point. Patient data collected included age, gender, ITP diagnosis/remission dates, and results of DATs, plasma bilirubin levels, and all plasma LDH levels with concurrent platelet counts measured while ITP was active (remission defined as platelet count ≥100 × 109/l off ITP-directed treatment for ≥12 months without relapse). The utilized LDH assay (Roche Diagnostics, Rotkreuz, Switzerland) was last modified in 1988, 2 years prior to the study data collection start date. At that time our normal institutional reference range (110–210 μ/l) was developed utilizing plasma specimens from 241 healthy blood bank donors [mean LDH 155 μ/l, standard deviation (SD) 27 μ/l]. This reference range has not changed since (confirmed by interval healthy subject reassessment) through intermittent laboratory equipment upgrades. Statistical analysis was performed using Stata version 14.2 (StataCorp LLC, College Station, TX), GraphPad Prism 7 (GraphPad, Inc., San Diego, CA) and Microsoft Excel 2016 (Microsoft Corp., Redmond, WA).
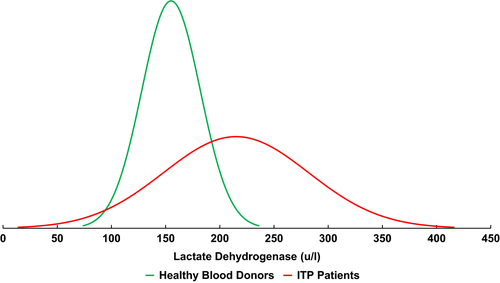
Of 361 ITP patients screened, 182 qualified for study inclusion. Median age at ITP diagnosis was 53 (range, 19–90), 60% of patients were female, median time from ITP diagnosis was 12·6 years (range, 1·1–61·3 years) and 20·3% of patients had achieved remission. A total of 2038 plasma LDH assays were performed in these 182 patients (median 5 per patient); 1959 assays had paired platelet counts from the same blood draw. Fifty-three percent of all LDH assays were elevated above the reference range. Sixty-five percent of patients had at least one elevated LDH value and individual patient median LDH was elevated in 43%. Individual patient median LDH values for the cohort were normally distributed with a mean of 215 μ/l [95% confidence interval (CI), 205–224 μ/l] and SD of 67 μ/l. Figure 1 demonstrates the difference in Gaussian distributions between the 182 ITP patients and 241 healthy subjects. The means of the two groups were significantly different (P < 0·0001 by two-tailed t-test with Welch correction). The difference in the means was 59 μ/l (95% CI, 49–70 μ/l), more than two times the standard deviation of the healthy subject population (27 μ/l).
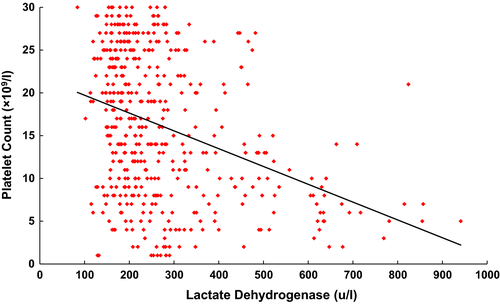
There was no relationship between LDH elevation and patient age, gender or attainment of remission in a multivariable logistic regression model. There was an inverse correlation between LDH level and platelet count. Using a platelet count threshold of ≤100 × 109/l, we found a statistically significant inverse correlation between LDH level and platelet count (r = −0·17, P < 0·0001). The correlation strengthened as the platelet count threshold dropped (threshold ≤50 × 109/l, r = 0·19, P < 0·0001; threshold ≤30 × 109/l, r = 0·37, P < 0·0001). This correlation, probably driven by the patients with the most severe thrombocytopenia and LDH elevation, is demonstrated in Fig 2.
Utilizing strict criteria for patient inclusion to minimize potential confounding factors, we demonstrated that plasma LDH is frequently elevated in ITP patients, with a mean LDH (215 μ/l) in ITP patients higher than the reference range upper limit (210 μ/l) derived from 241 healthy subjects. We additionally found a statistically significant inverse correlation between LDH value and concurrently drawn platelet count, suggesting LDH elevation is occurring at least partially due to increased platelet turnover. The correlation’s relative weakness and its strengthening with lower platelet counts may be due to variation in predominant pathophysiological mechanisms between patients (increased platelet destruction versus decreased platelet production), a non-linear relationship between platelet turnover rate and platelet count (Heyns Adu et al, 1986) or a confounding effect of effective treatment emerging at higher counts. Prospective evaluation could address the latter issue, as accurate exclusion of LDH measurements while on treatment was not feasible in this retrospective study drawing upon 28 years of data. Additional study could also compare remission LDH values to active disease values from the same patients. This was not possible in this study because only a small number of the patients who achieved remission had post-remission LDH values available.
Recognition that approximately half of ITP patients have mild-to-moderate LDH elevation is important during diagnostic evaluation of thrombocytopenia. While LDH assays vary considerably between institutions, the magnitude of LDH elevation in ITP patients relative to healthy controls (approximately a mean 40% higher, Fig 1) may be consistent between centres (and requires validation). This knowledge could prevent expensive and/or unnecessary diagnostic searches for alternative diagnoses. In addition, LDH isozyme testing may be a useful diagnostic test for ITP. Platelets have a specific LDH isozyme signature (Hule, 1966; Knudsen & Gormsen, 1968; Schneider et al, 1968), so specific patterns may be detectable in ITP patient plasma. This is an ideal topic for further investigation.
Acknowledgements
This study was approved by the Institutional Review Board (approval 2018P000964/PHS) of the Massachusetts General Hospital. H. Al-Samkari is the recipient of the National Hemophilia Foundation-Shire Clinical Fellowship Award, which provides partial salary support. The authors would like to thank Dr. James Flood, director of the clinical chemistry lab at the Massachusetts General Hospital, for his invaluable consultation and for keeping over 30 years of detailed records.
Authorship Contributions
H. Al-Samkari designed the study, collected data, analysed data, created tables and figures, and wrote and revised the manuscript; D. Kuter designed the study, critically revised the manuscript and supervised the study.
Disclosures
The authors report no disclosures relevant to the submitted work. Universal Disclosures: Al-Samkari: Consultancy (Agios, Dova, Moderna), Research funding (Agios, Dova). Kuter: Research funding (Protalex, Bristol-Myers Squibb, Rigel, Bioverativ, Agios, Syntimmune, Principia, Alnylam); Consultancy (ONO, Pfizer, 3SBios, Eisai, GlaxoSmithKline, Genzyme, Shire, Amgen, Shionogi, Rigel, Syntimmune, MedImmune, Novartis, Alexion, Bioverativ, Argenx, Zafgen, Fujifilm, Principia, Kyowa Kirin, Takeda, Platelet Disorders Support Group).