Deferasirox in the management of iron-overload in patients with myelofibrosis: a multicentre study from the Rete Ematologica Lombarda (IRON-M study)
Myelofibrosis (MF) is a clonal myeloproliferative neoplasm with a high prevalence of transfusion-dependent (TD) anaemia, which may lead to iron overload (IOL) (Tefferi et al, 2012). At present, very few data are available on the role of iron chelation therapy (ICT) with deferasirox (DFX) in MF (Elli et al, 2014; Latagliata et al, 2015). In this study, we assessed the efficacy of DFX, in terms of iron chelation response (ICR), haematological improvement (HI), impact on survival and leukaemic evolution, on 45 consecutive MF patients fulfilling the inclusion criteria (Data S1).
The study (IRON-M) was developed within the Rete Ematologica Lombarda, a network of Haematology units in Lombardia, and approved by each Institutional Review Board. Demographics features at start of DFX (baseline) and the methods for the assessment of IOL are available in Table SI and Data S1, respectively.
Patients received DFX after a median MF duration of 26·8 months [interquartile range (IQR) 2·6–208·7] with a TD latency of 12·5 months (IQR 1·2–146·1). The median starting dose of DFX was 10 mg/kg/day (IQR 6·25–25·4). At baseline, 18 patients (40%) were cytoreduction-naïve and 27 (60%) on therapy (hydroxycarbamide in 12, ruxolitinib in 14 and other therapy in 1). Forty-one of 45 patients (91·1%) were evaluable for DFX response (>3 months of treatment). Regarding chelation efficacy, ICR was defined as a stable ferritin level below 1000 μg/l or a stable reduction exceeding 50% of the baseline. After a median DFX-exposure of 17·2 months (IQR 3–59·5), 12 patients (29·3%) obtained ICR.
The principal variables at baseline according to ICR are reported in Table 1. Patients who obtained ICR had significantly lower ferritin values (1390 μg/l vs. 2000 μg/l, P = 0·0018), lower transfusion burden of red blood cells (RBC) units pre-ICT (16 vs. 31 units transfused; P = 0·032) and lower exposure to TD pre-ICT (8·7 months vs. 16·7 months, P = 0·04), when compared to those who did not.
| Variable | Non-responders (n = 29) | Responders (n = 12) | P-value |
|---|---|---|---|
| Median age (years) | 71·4 | 75·2 | 0·752 |
| Gender (M/F), n (%) | 18/11 (62·1%/37·9%) | 7/5 (58·3%/41·7) | 0·823 |
| Myelofibrosis, n (%) | |||
| Primary | 20 (69) | 7 (58·3) | 0·514 |
| Secondary | 9 (31) | 5 (41·7) | |
| Driver mutations, n (%) | |||
| JAK2 V617F | 24 (82·8) | 11 (91·7) | 0·511 |
| CALR | 3 (10·3) | 0 (0) | |
| MPL | 0 (0) | 0 (0) | |
| Triple-negative | 2 (6·9) | 1 (8·3) | |
| DIPSS, n (%) | |||
| Low/intermediate-1 | 3 (10·3) | 0 (0) | 0·383 |
| High/intermediate-2 | 26 (89·7) | 12 (100) | |
| Median ferritin level (μg/l) | 2000 | 1390 | 0·0018 |
| Median full blood count | |||
| Hb (g/l) | 86 | 87 | 0·501 |
| WBC (×109/l) | 8·5 | 7·6 | 0·641 |
| PLT (×109/l) | 119 | 84 | 0·685 |
| Number of RBC units/patient received (median) | 31 | 16 | 0·032 |
| Median starting dose of DFX (mg/kg/day) | 10 | 10·5 | 0·374 |
| Median time from diagnosis to baseline (months) | 31·7 | 17·2 | 0·160 |
| Median time from transfusion start to baseline (months) | 16·7 | 8·7 | 0·04 |
| Median time of DFX exposure (months) | 11·2 | 18·1 | 0·228 |
| Median ferritin level (μg/l) after ICT start | |||
| at 6 months | 2145 | 1099 | 0·0016 |
| at 12 months | 2236 | 906·5 | 0·0008 |
| at 18 months | 2853 | 867 | 0·0006 |
| at 24 months | 2963 | 1221 | 0·021 |
- Significant P-values are shown in bold.
- DFX, deferasirox; DIPSS, Dynamic International Prognostic Scoring System; F, female; Hb, haemoglobin; M, male; PLT, platelet count; RBC, red blood cell; WBC, white blood cell count.
As expected, ICR patients showed a progressive significant reduction of ferritin levels at 6, 12 and 18 months, with respect to baseline values (Fig S1).
The International Working Group criteria (Cheson et al, 2006) were applied to assess HI during ICT (Data S2). Erythroid response (ER) was defined as complete (CR: achievement of transfusion independency), partial (PR: reduction in the transfusion requirement and/or increase of haemoglobin levels) or no response (NR). ER was achieved in 18/41 patients (43·9%) with seven (17%) obtaining CR, 11 (26·8%) PR and 23 (56·1%) NR. Achieving ICR predicted ER: 11 (91·7%) patients with ICR obtained CR or PR compared to 7 (24·1%) without ICR (P < 0·001).
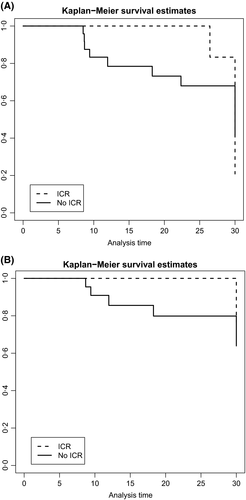
After a median follow-up of 52·8 months (IQR 14·4–267·2) from MF diagnosis, 17 (41·5%) patients had died, eight of them following leukaemic evolution or disease progression. For survival analysis, a landmark analysis including patients alive at 6 months of DFX therapy was performed (Data S3). At 2 years, survival from DFX initiation was 100% in patients with ICR and 70% in those without [hazard ratio (HR) 0·7, 95% confidence interval (CI): 46·5–84·8%; P = 0·007, Fig 1A]. The rate of leukaemic evolution or disease progression was higher in patients without ICR than in those with ICR (1·5 vs. 0·6 events/person-year) with a significantly different 2-year leukaemia-free-survival (LFS) between two groups (81·6% vs. 100%, HR 0·81, 95% CI: 57·4–92·8%; P = 0·039, Fig 1B). This difference in terms of LFS at 2 years was even more evident in patients with any ER as compared those with NR (P = 0·024).
Of 45 patients evaluable for toxicity, 20 (44·4%) experienced extra-haematological adverse events (Table SII). Overall, a dose reduction/temporary discontinuation related to drug toxicity was reported in 17 (37·7%); however only 11 (24·4%) patients completely discontinued ICT because of grade ≥ 2 toxicity.
Our preliminary data open new insights regarding the benefit of DFX in MF patients with TD anaemia. Several emerging lines of evidence indicated that DFX can improve haematopoiesis, however, these data come from single case descriptions (Di Tucci et al, 2007; Piro et al, 2018) or small retrospective studies (Elli et al, 2014; Latagliata et al, 2015). DFX, probably independently of its iron-chelating property but through the interference on its reactive oxygen species (ROS) signalling activation, may influence key factors involved in self-renewal/differentiation of haematopoietic stem cells (HSC; Zhang et al, 2015). It is known from recent studies that ROS balance may determine the destiny of stem cells; exceedingly high ROS levels, as may occur during chronic inflammation or IOL, can promote stem cell exhaustion and subsequent apoptosis. Inadequate ROS homeostasis resulting in oxidative stress and genetic instability in HSC and myeloid progenitors, could explain the role of “bone marrow iron toxicity” in the clonal evolution phenomenon (Zhang et al, 2015; Isidori et al, 2018). DFX is a potent nuclear factor-κB inhibitor, and it acts by reducing oxidative stress that can be linked to an anti-proliferative effect (Pilo & Angelucci, 2018).
The benefit on survival and LFS we found in patients who obtained ICR parallels the biological observation of a potential correlation between an efficient chelation with DFX and a reduction in oxidative stress and genetic instability in HSC and myeloid progenitors (Isidori et al, 2018).
As this is a retrospective study, we cannot definitively exclude an impact of the patient's disease characteristics on best outcomes of ICR group. However, we found that the distribution of the Dynamic International Prognostic Scoring System risk categories, as well as the mutational status in patients responding or not responding to DFX, was similar. In addition, our results suggest that starting DFX early in disease evolution is of benefit for patients: in fact, patients who started ICT with lower baseline ferritin level or lower transfusion burden were more likely to respond.
In conclusion, the present multicentre study reported a large retrospective analysis of DFX in the management of IOL in MF. We showed that DFX treatment is feasible and effective in the MF setting.
Further controlled prospective trials are required to confirm a positive impact of ICT with DFX on outcome of disease.
Funding
FP was supported by grants of the Fondazione Regionale Ricerca Biomedica, Milan, Italy [FRRB project no. 2015-0042, Genomic profiling of rare haematological malignancies, development of personalized medicine strategies, and their implementation into the Rete Ematologica Lombarda (REL) clinical network], by Fondazione Matarelli (Milano, Italy), Fondazione Rusconi (Varese, Italy) and AIL Varese ONLUS.
Conflict of interests
All other authors declare no conflict of interest.
Author's contributions
EME and PF: designed the study and wrote the paper. LA, EME and AA: performed the statistical analysis. All Authors: collected clinical, laboratory, molecular and histology data. All authors gave final approval to the manuscript.




