The use of viscoelastic haemostatic assays in the management of major bleeding
A British Society for Haematology Guideline
Major haemorrhage is an important cause of morbidity and mortality, affecting up to 40% of all trauma patients (Stanworth et al, 2016) and 10% of cardiac surgery patients (Serraino & Murphy, 2017). Blood loss is one of the main causes of morbidity following liver transplantation (Gurusamy et al, 2011) and is one of the most common causes of death worldwide in women at the time of delivery (Say et al, 2014). Diagnosis of major bleeding is difficult and is often made using clinical measures (e.g. rising heart rate, falling blood pressure) but these measures can be insensitive, particularly in younger patients in whom blood loss can be masked and haemodynamic stability preserved or in elderly patients on cardiovascular modulating medication. Detection and correction of coagulopathy is therefore an important aspect of management of severe haemorrhage.
The British Society for Haematology (BSH) guidelines (Hunt et al, 2015a) recommend the use of serial standard laboratory tests (SLTs), taken every 30–60 min, to monitor major haemorrhage in most clinical settings. There are, however, inherent difficulties with SLTs. Average turn-around-times are between 27 (Cotton et al, 2011) and 77 min (Davenport et al, 2011) which is often too slow for a rapidly evolving situation. Furthermore, the ability of SLTs to predict major bleeding and therefore allow pre-emptive treatment is limited (Segal & Dzik, 2005; Davenport et al, 2011). In liver transplantation for example, it is well known that the prothrombin time (PT) or the International Normalised Ratio (INR) does not differentiate between patients who will or will not bleed excessively (Massicotte et al, 2014), nor does an elevated INR exclude underlying hypercoagulability (Krzanicki et al, 2013).
Viscoelastic haemostatic assays (VHA) are increasingly being used during the management of major bleeding. There are several recent comprehensive systematic reviews evaluating the available evidence (Whiting et al, 2015; Fahrendorff et al, 2017; Serraino & Murphy, 2017). Current National Institute for Health and Care Excellence (NICE) guidance recommends VHA use during cardiac surgery, but not for obstetric or trauma haemorrhage (Whiting et al, 2015). This BSH guideline recognises the limited available evidence but, within these constraints, aims to provide pragmatic and practical advice to practising clinicians as to how to interpret and use VHA results during the management of major bleeding in four common scenarios: obstetric haemorrhage, liver disease, cardiac surgery and trauma haemorrhage.
Methodology
This guideline was compiled according to the BSH process at www.b-s-h.org.uk/guidelines. Details of the methodology for inclusion of studies, including the PRISMA diagram, can be found in the online supplement (Appendix S1). Grading of Recommendations Assessment, Development and Evaluation (GRADE) nomenclature was used to evaluate levels of evidence and to assess the strength of recommendations. The GRADE criteria can be found at http://www.gradeworkinggroup.org.
Review of the manuscript
Review of the manuscript was performed by the BSH Guidelines Committee Haemostasis and Thrombosis Task Force, the BSH Guidelines Committee and the Haemostasis and Thrombosis sounding board of the BSH. It was also placed on the members section of the BSH website for comment. The Association of Anaesthetists of Great Britain and Ireland endorsed the document.
Machine methodology, quality assurance and test accuracy
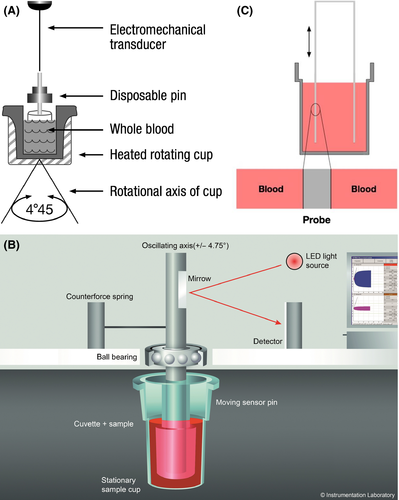
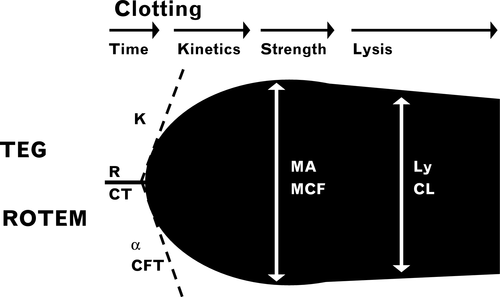
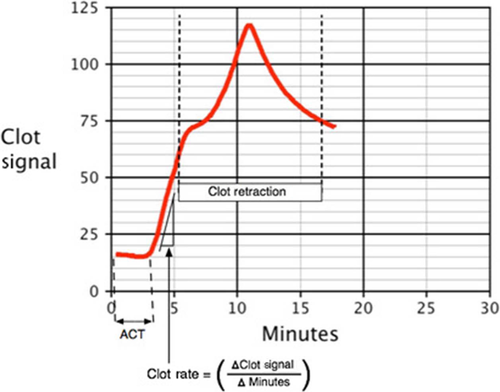
Viscoelastic tests include thromboelastography (TEG), thromboelastometry (ROTEM) and Sonoclot. The most widely used machines are: TEG5000 (Haemonetics S.A., Signy, Switzerland), ROTEM delta (TEM International, GmbH, Munich, Germany) and Sonoclot (Sienco Inc, Morrison, CO, USA). Until recently, all three devices used similar principles based on a cup and pin method to measure the mechanical properties of clot formation in whole blood (Fig 1). A variety of activators are used for each device to examine different aspects of the haemostatic system (Tables 1-3) and, as the blood clots, a graphical representation is made. A typical trace for TEG and ROTEM is shown in Fig 2 and a Sonoclot trace is illustrated in Fig 3. Although TEG and ROTEM traces look identical, the parameters are not directly interchangeable and should not be regarded as equivalent (Coakley et al, 2006; Venema et al, 2010; Hagemo et al, 2015; Rizoli et al, 2016).
| TEG 5000 (cup and pin method) | TEG 6s (cartridge method) | What the trace looks at: | |
|---|---|---|---|
| Sample type |
Fresh WB Citrated WB |
Citrated WB | – |
| Tests available |
‘Plain cup’: Kaolin |
CK: Kaolin, Ca |
Standard clot formation – activating the intrinsic pathway |
|
‘Heparinase cup ’: Kaolin, heparinase |
CKH: Kaolin, heparinase, Ca |
When compared to a standard kaolin activated trace a shorter R time suggests the presence of heparin | |
|
rTEG: Kaolin, TF |
CRT: Kaolin, TF, Ca |
Standard clot formation activating both intrinsic and extrinsic pathways (particularly helpful for major haemorrhage and rapid results) | |
|
FF: TF, Reopro |
CFF: TF, Reopro, Ca |
Platelet inhibitor added: contribution of fibrinogen to clot remains. When trace is compared to a standard kaolin trace the platelet contribution can be estimated |
- The words in italics indicate the names given to the assays by the manufacturer. The reagents used in these assays are described beneath each italicised name.
- Ca, calcium chloride; CFF, citrated functional fibrinogen; CK, citrated kaolin; CKH, citrated kaolin and heaprinase; CRT, clot retention time; FF, functional fibrinogen; R time, reaction time; Reopro, a GPIIb/IIIa inhibitor, inhibiting platelet activity; rTEG, rapid TEG; TEG, thromboelastography; TF, tissue factor; WB, whole blood.
| Gamma and delta (cup and pin method) | Sigma (cartridge method) | What the trace looks at: | |
|---|---|---|---|
| Sample type | Citrated WB | Citrated WB | – |
| Tests available |
INTEM: Ellagic acid |
‘ROTEM sigma complete cartridge’: Includes: FIBTEM, EXTEM, INTEM and APTEM channels |
INTEM: Standard clot formation – activating the intrinsic pathway |
|
EXTEM: TF |
‘ROTEM sigma complete +hep cartridge’: Includes: FIBTEM, EXTEM, INTEM and HEPTEM channels |
EXTEM: Standard clot formation – activating the extrinsic pathway | |
|
HEPTEM: Ellagic acid, heparinase |
HEPTEM: When compared to an INTEM trace a shorter CT time suggests presence of heparin | ||
|
FIBTEM: TF + cytochalasin C |
FIBTEM: Platelet inhibitor added: contribution of fibrinogen to clot remains. Can compare results to EXTEM trace to get idea of platelet contribution |
||
|
APTEM: TF + aprotinin |
APTEM: Aprotinin added: trace compared to EXTEM, differences in MA/MCF suggest contribution of fibrinolysis |
- The words in italics indicate the names given to the assays by the manufacturer. The reagents used in these assays are described beneath each italicised name.
- CT, clotting time; MA, maximum amplitude; MCF, maximal clot formation; TF, tissue factor; WB, whole blood.
| Sonoclot | What the trace looks at: | |
|---|---|---|
| Sample type | Fresh WB, Citrated WB, plasma | |
| Tests available |
kACT: Kaolin |
Used to manage heparin therapy (with/without aprotinin) |
|
Son ACT: Celite |
Used to manage heparin therapy (without aprotinin) | |
|
aiACT: Celite, clay |
Used to manage heparin therapy, with aprotinin | |
|
gbACT+: Glass beads |
Standard clotting assessment and platelet function for use in non-heparinised patients | |
|
H-gbACT+: Glass beads + heparinase |
Standard clotting assessment and platelet function for use in heparinised patients |
- The words in italics indicate the names given to the assays by the manufacturer. The reagents used in these assays are described beneath each italicised name.
- aiACT, aprotinin insensitive activated clotting time; gbACT, glass bead-activated clotting time; H-gbACT, heparinise glass bead-activated clotting time; kACT, kaolin-based activated clotting time; Son ACT, time until fibrin formation.
One of the major drawbacks of VHA machines has been the need for users to be trained in basic pipetting: the TEG5000 and Sonoclot operate with manual pipetting, the ROTEM delta with automated pipetting. In response to this challenge, the TEG and ROTEM manufacturers have developed cartridge-based techniques designed to improve ease of use and precision – the TEG6s and the ROTEM sigma.
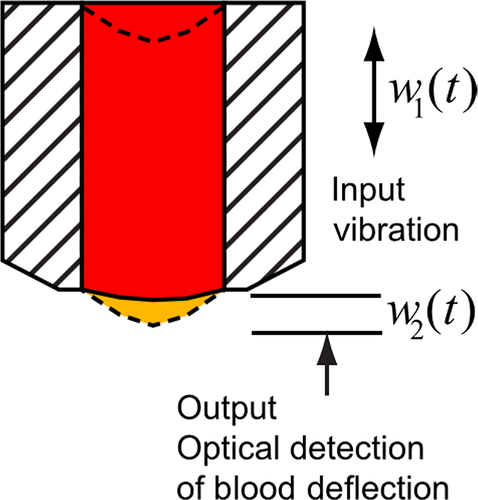
TEG6s has microfluidic cartridges pre-loaded with reagents and uniquely uses resonance technology to record clot formation (Fig 4). Each cartridge has four channels, containing different reagents (Table 1). Despite a different methodology, observational data have shown good correlation between TEG5000 and TEG6s (R time, K time, alpha angle) (Gurbel et al, 2016) in 157 healthy volunteers and 300 cardiac patients. But, similarly to all VHA devices, theTEG6s remains sensitive to vibration (Gill, 2017), an important consideration for road- or air-based analysis. TEG6s cartridges to detect anti-thrombin and anti-Xa agents are in development and a pilot study (Bliden et al, 2017) showed high sensitivity and specificity for direct oral anticoagulant (DOAC) therapies.
Only one study has compared ROTEM sigma and delta, evaluating EXTEM and FIBTEM in 30 pregnant volunteers. This study showed no differences between FIBTEM A5 values but reported significantly lower EXTEM A5 and shorter EXTEM clotting time (CT) values with the sigma (Crighton et al, 2017). Further comparative data are needed for exploring new versus older technologies, as switching devices may require significant adjustments to the corresponding VHA-based transfusion algorithm.
Sample type and pre-analytical issues
Native whole blood samples need immediate analysis. Anticoagulated samples should be analysed within four hours of venesection. No specific rest period is recommended by manufacturers prior to analysing citrated blood samples. Users of ROTEM devices have variously recommended resting samples between 30 (Andreasen et al, 2011; Armstrong et al, 2011) and 120 (Theusinger et al, 2010) minutes, whilst others report immediate testing (Oswald et al, 2010; Ogawa et al, 2012a,b).
Pragmatically, it is reasonable to perform immediate testing for all VHA tests.
Precision and accuracy of testing
Precision of VHA testing has not been widely reported, although operator variation seems to have an important effect. Coefficients of variation of 2·6–11·2% for ROTEM delta and 7·4–19% for TEG5000 parameters were reported in one study with seven operators (Anderson et al, 2014). Inter-laboratory comparison with external quality assurance (EQA) samples shows much poorer precision: 7–49% for TEG5000 and 7–83% for ROTEM delta (Kitchen et al, 2010). Notably this EQA programme used lyophilised citrated plasma samples, which may explain the wide variability. There are no EQA data for the TEG6s or ROTEM sigma.
Internal quality control
There is no consensus on the desirable frequency of Internal Quality Control (IQC) – reports have ranged from eight-hourly (Pommerening et al, 2014) to weekly (Jeger et al, 2012). As with all IQC, this methodology checks the quality control of the user, and the reagents as well as the device. TEG manufacturers recommend daily electronic checks and monthly IQC for low volume users and more frequent analysis for higher users. ROTEM recommends weekly quality control checks and Sonoclot recommends a viscosity check daily and a monthly reference plasma quality control. These are minimum recommendations and users should take into account volume of testing when deciding on frequency of IQC.
External quality assurance
The UK National External Quality Assurance Scheme (NEQAS) for Blood Coagulation programme provides a VHA EQA service for TEG5000 and ROTEM delta which uses lyophilized citrated plasma samples with plans in place to offer EQA for TEG6s and ROTEM sigma. The External quality Control of diagnostic Assays and Tests (ECAT) Foundation also offers an EQA scheme using lyophilised plasma.
Reference ranges
Viscoelastic haemostatic assays tests are poorly standardised and, apart from manufacturers’ reported reference ranges, there is no published consensus for normal ranges. Hospitals should therefore determine local references ranges as is standard practice for the majority of laboratory tests. Determining local ranges may not be practical in the paediatric setting – reference ranges have been reported for children using the ROTEM delta (Oswald et al, 2010) and the TEG5000 (Chan et al, 2007).
Practice points
- Reference ranges should be determined locally and re-established when a new machine is introduced.
- For non-cartridge based methods, staff should be trained and have good pipetting technique. Training and competency should be documented.
- Anticoagulated samples should be tested within four hours; no resting period is required.
- Internal Quality Control (IQC) should be performed daily for high volume usage or weekly if low volume usage.
- Thromboelastography (TEG) and thromboelastometry (ROTEM)measures must not be used interchangeably.
- Participation in an accredited external quality assurance (EQA) programme is recommended.
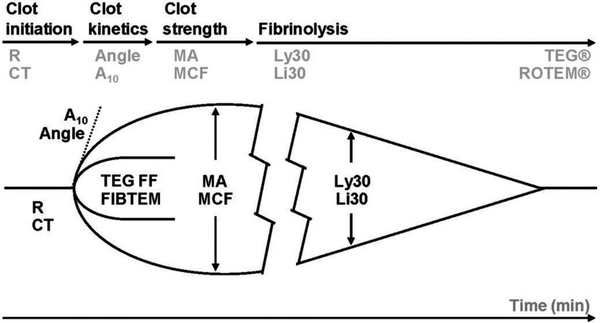
VHA traces
- Clot initiation [R (reaction) time - TEG5000; ACT (activated clotting time) – rapid TEG or rTEG; CT (clotting time) – ROTEM devices]: is broadly similar to the PT or activated partial thromboplastin time (aPTT) and measures from test start until fibrin begins to be formed. Warfarin and heparin prolong this measurement, as can any other significant hypocoagulability, including low fibrinogen concentration.
- Fibrin polymerisation [K (kinetics) time, α angle – TEG devices; CFT (clot formation time), α angle – ROTEM devices]: reflects the speed at which fibrin is formed and how well it binds to platelets. The value is dependent on sufficient fibrinogen and platelet number and function.
- Clot strength [MA (maximum amplitude) – TEG devices; A (amplitude) and MCF (maximal clot firmness) – ROTEM devices]: these measures make up most of the trace and are a combined assessment of fibrinogen and platelet interaction. To differentiate their effects the standard VHA trace should be compared with a fibrinogen trace (ROTEM – FIBTEM; TEG – functional fibrinogen (ff)) in which the contribution of platelets is removed by adding a platelet inhibitor. Factor XIII also contributes to a small degree to clot strength.
- Lysis of the clot [LY30 (lysis at 30 min) – TEG devices; ML (maximal lysis) – ROTEM devices]: some clot strength diminution is expected by the end of a VHA trace, as platelet retraction is a normal phenomenon. Cut-offs are given, specific to each device, and fibrinolysis is evident if these are exceeded. Although TEG and ROTEM are able to detect increased lysis, they are insensitive to mildly and or moderately increased fibrinolysis and should not be used to confirm its absence (Raza et al, 2013) nor should it be used as a reason to withhold tranexamic acid.
Viscoelastic haemostatic assays tests are generally insensitive to anti-platelet agents (except α2bβ3 blockers) (Tynngård et al, 2015). Standard ROTEM tests (EXTEM/INTEM CT) can detect DOACs (dabigatran, edoxaban, rivaroxaban) at therapeutic levels, but appear insensitive to apixaban (Seyve et al, 2018). Sonoclot measures are set out in Fig 3.
Obstetric and postpartum bleeding
Viscoelastic haemostatic assays are most useful in major obstetric bleeding when the obstetric haemorrhage protocol is activated, though tests may be run earlier if coagulopathy is expected, e.g., with placental abruption or amniotic fluid embolism.
The coagulation system at term is procoagulant and normal ranges for many TEG and ROTEM parameters differ at delivery from non-pregnant values. On the ROTEM delta, CTs are shorter and EXTEM and FIBTEM A5 (amplitude at 5 min), A10 and MCF are higher for women in the third trimester of pregnancy (Armstrong et al, 2011; Oudghiri et al, 2011; van Rheenen-Flach et al, 2013; de Lange et al, 2014). On the TEG5000, R and K times are shorter and alpha angle and MA higher at term (Polak et al, 2011; Della Rocca et al, 2012). This means that an MA or MCF that is normal for the non-pregnant population may suggest a developing coagulopathy in a term woman. An abnormal ROTEM or TEG trace may help to alert clinicians to the possibility of an amniotic fluid embolus or placental abruption. There are no published data on the normal range for Sonoclot during pregnancy.
Prediction of bleeding/coagulopathy
FIBTEM and EXTEM measured on admission to the delivery suite or before bleeding starts are not predictive of future bleeding (Kaufner et al, 2017). However, a low FIBTEM A5 and MCF, taken at the time of moderate postpartum haemorrhage (1000–1500 ml), is predictive of the need for transfusion of ≥4 units red blood cells (RBC) [Receiver operator characteristic area under the curve (ROC AUC) (95% confidence intervals (CI)) 0·78 (0·69–0·88)] and bleeds >2500 ml [0·75 (0·66–0·85)]. Women who progressed to ≥4 units RBC had a FIBTEM A5 of 13 mm [interquartile range (IQR): 10–17] whereas those that did not progress had a FIBTEM A5 of 19 mm (17–23) (Collins et al, 2014). TEG clot strength measures, using an MA cut -off of 40 mm (kaolin TEG5000 MA) are also predictive of major obstetric haemorrhage: ROC AUC (95% CI) 0·9 (0·83–0·95) (Barinov et al, 2017). There are no published data on the role of Sonoclot for predicting progression of obstetric bleeding.
Diagnosis of bleeding/coagulopathy
Clauss fibrinogen and FIBTEM A5/MCF correlate moderately well during postpartum haemorrhage (PPH) and can be used to identify women with a reduced Clauss fibrinogen (Huissoud et al, 2009). A double blind, randomised, placebo-controlled trial of women experiencing moderate to severe PPH showed that a FIBTEM A5 >12 mm indicates a fibrinogen level adequate for haemostasis (Collins et al, 2017a). An observational study showed that the combination of an EXTEM CT <100 s and FIBTEM A5 >12 mm was associated with adequate haemostasis (Mallaiah et al, 2015a,b).
Tranexamic acid improves outcomes during PPH if infused within 3 h of delivery (WOMAN Trial Collaborators, 2017). There are no data to support the use of APTEM or TEG clot lysis parameters to guide tranexamic acid infusion. Normal APTEM or TEG clot lysis results should not be used to withhold tranexamic acid.
There are no published data to link the platelet count with ROTEM or TEG parameters during PPH. Algorithms developed for other causes of bleeding may not be applicable in this situation because clot strength parameters are raised at the time of delivery.
Use of ROTEM/TEG/Sonoclot for guiding transfusion and haemostatic therapy
NICE guidance does not support the use of VHAs to guide blood component replacement during PPH and recommends further studies (Whiting et al, 2015).
A prospective, double-blind study randomised women with moderate to severe PPH and a FIBTEM A5 ≤15 mm to fibrinogen concentrate or placebo (n = 55). No reduction in transfusion requirements or blood loss was reported. Subgroup analysis suggested that if the FIBTEM A5 was ≥12 mm fibrinogen infusion was not required (Collins et al, 2017a). In the observation arm of the same study, 605 women were not infused with fresh frozen plasma (FFP) if FIBTEM A5 was >15 mm or bleeding had stopped. This did not result in any women developing clinically significant haemostatic impairment, defined as continuing bleeding associated with a PT or aPTT >1·5 times normal (Mavrides et al, 2016; Collins et al, 2017a,b).
An observational study showed that infusing fibrinogen concentrate when FIBTEM A5 was <7 mm, or <12 mm with severe bleeding, and infusing FFP when CT was >100 s (n = 107) reduced RBC usage, transfusion-associated fluid overload and admission to the intensive care unit (ICU) compared to the use of empirical transfusion with RBC:FFP:platelets in a ratio of 4:4:1 units (n = 42) in a before and after study (Mallaiah et al, 2015a,b).
An observational study compared standard care (n = 29) with early surgical intervention, mechanical uterine pressure plus TEG-guided transfusion (n = 90) (Barinov et al, 2017). There was a statistically significant reduction in hysterectomy, total blood loss and FFP transfusion in the combined strategy group. The report does not state what TEG parameters were used to trigger FFP or platelet transfusion. It not possible to conclude which of the interventions contributed to the improved outcomes (Barinov et al, 2017). A TEG-based algorithm for managing obstetric bleeding has been published but no data were presented on whether the algorithm affected outcomes (Hill et al, 2012).
There are no published data on the role of Sonoclot to guide blood component use during PPH.
Recommendations
- Viscoelastic haemostatic assays (VHA) are not usually helpful for predicting post-partum haemorrhage when taken during labour in a non-bleeding pregnant woman. Grade 2C.
- VHA may be used as part of an agreed algorithm to manage postpartum haemorrhage when the local institution's major obstetric haemorrhage protocol is activated. Grade 2C.
- During ongoing major postpartum haemorrhage, if the FIBTEM A5 is >12 mm fibrinogen replacement is unlikely to improve clinical haemostasis. Grade 2B.
- During major postpartum haemorrhage, if FIBTEM A5 is <7 mm, or <12 mm with ongoing bleeding, fibrinogen replacement may improve clinical haemostasis. Grade 2C.
- In a bleeding pregnant or post-partum patient, tranexamic acid should not be withheld based on the thromboelastography (TEG) or thromboelastometry (ROTEM)parameters. Grade 1B.
Liver disease and Liver surgery
Prediction of bleeding/coagulopathy
Viscoelastic haemostatic assays have a better predictive value for bleeding in liver disease (Fayed et al, 2015; Tafur et al, 2016; Pustavoitau et al, 2017) and re-bleeding than SLTs (Chau et al, 1998). A retrospective, single centre study comparing SLT and ROTEM in post-operative bleeding in adult liver transplant (LT) patients found that several ROTEM parameters predicted bleeding (cut-offs shown in brackets): EXTEM CT (≥65 s), INTEM CFT (≥181 s), FIBTEM A10 (≤13 mm) and FIBTEM MCF (≤15 mm) (AUC 0·682, 0·615, 0·615 and 0·611 respectively) compared to no SLT tests (Dotsch et al, 2017). In cirrhotic patients, ROTEM values were associated with bleeding, specifically, reduced EXTEM MCF (median values bleeding vs non-bleeding: 38 mm vs. 43 mm) and FIBTEM MCF (8 mm vs. 13 mm), and these values were associated with lower factor XIII levels (Bedreli et al, 2017).
A prospective observational study in 263 LT patients with transfusion thresholds based on SLTs, found the best threshold that predicted platelet transfusion was A10 (EXTEM) ≤35 mm and, for fibrinogen, an A10 (FIBTEM) ≤8 mm (Blasi et al, 2012), with a negative predictive value (NPV) and positive predictive value (PPV) of 95% and 27%, respectively. A retrospective analysis of ROTEM datasets in 239 LT patients concluded that the EXTEM and FIBTEM A5 measures can be used to guide therapy (Song et al 2014) with an EXTEM A5 <27 mm predicting platelet count <50 × 10−9 per litre and FIBTEM A5 <5 mm predicting fibrinogen <1·0 g/l. Patients with values above these ranges were unlikely to bleed.
Diagnosis of bleeding/coagulopathy
Standard laboratory tests frequently indicate a hypocoagulable state in patients with liver disease, whereas VHAs exhibit a spectrum from hypo- to hypercoagulable. In a cohort of 273 patients with cirrhosis, TEG parameters were within normal limits, although the MA decreased in proportion to the severity of liver disease and degree of thrombocytopenia (Stravitz, 2012). A good correlation has been reported between the MCF and platelet count and fibrinogen levels (Roullet et al, 2010). But, although TEG ff and FIBTEM correlate well with Clauss fibrinogen, they can overestimate low levels (<1·0 g/l), especially at the time of graft reperfusion, and should be interpreted with caution (Yang Lu et al, 2014).
Hyperfibrinolysis is a significant cause of non-surgical bleeding in LT, with the highest incidence occurring immediately after graft reperfusion. A prospective observational study in 37 LT comparing ROTEM and TEG (Abuelkasem et al, 2016) showed that tissue factor-triggered ROTEM tests were more sensitive than contact-activated kaolin TEG in identifying hyperfibrinolysis. [This study was limited by using VHA manufacturers’ definitions for lysis, without an additional gold standard measure (ML >15% ROTEM; Lysis30 >8% TEG)]. A heparin-like effect due to release of endogenous heparinoids at the time of graft reperfusion is commonly seen on VHA (Pivalizza et al, 1998) but whether this transient effect contributes to bleeding is controversial. It is recommended that TEG heparinase assays or HEPTEM are run immediately after graft reperfusion (Agarwal et al, 2008).
Use of ROTEM/TEG for guiding transfusion and haemostatic therapy
The perceived utility of TEG and ROTEM for intra-operative haemostatic monitoring has led to VHAs becoming a standard of care in many LT units, despite the lack of high quality data. The European Society of Anaesthesiologists guidelines recommend VHA use during LT (grade1C) (Kozek-Langenecker et al, 2017). From a practical stance, the major benefit is for patients with a high risk of bleeding [Model for End Stage Liver Disease score (MELD) >21 and /or expected difficult surgical dissection] and those poly-transfused intra-operatively. In this setting ROTEM has been shown to significantly reduce blood component use (P =< 0·05) and to reduce post-operative complications (Alamo et al, 2013).
Other observational studies have shown that VHA-based algorithms reduce transfusion during LT (Kang et al, 1985; Trzebicki et al, 2010) and increase the numbers of transfusion-free patients. One study showed a rise of non-transfused patients from 5% to 24% (P < 0·001), with concomitant reduction of massive transfusion from 13% to 2% (P < 0·005) (Leon-Justel et al, 2015). To date, the only RCT (n = 28) of TEG versus SLT demonstrated a significant reduction in FFP administration in the TEG group with a trend to less transfusion overall (Wang et al, 2010). A Cochrane review (Gurusamy et al, 2011) concluded that VHA may decrease blood loss and transfusion requirements in LT.
There are a number of treatment algorithms developed by different LT centres. The consensus in the LT community is not to correct VHA abnormalities unless there is active bleeding. The cut-off values that would trigger pre-emptive treatment have not been established and may lie significantly outside normal ranges (i.e. >15%) (Wang et al, 2012).
The clot strength is a composite measure of fibrinogen-platelet interaction, and VHA monitoring without assessment of fibrinogen can lead to increased transfusion of platelets (Larsen et al, 2011) with potentially detrimental effects (de Boer et al, 2008). Algorithms that include TEG ff or FIBTEM cut-offs for fibrinogen administration in patients who are actively bleeding increases the use of fibrinogen replacement but significantly reduces overall transfusion (Noval-Padillo et al, 2010; De Pietri et al, 2016). There is less information for thresholds to guide prothrombin complex concentrate (PCC) or FFP, but a retrospective review of 266 LT used a CT (EXTEM) ≥80 s as a threshold for PCC and a CT INTEM of ≥240 s for FFP transfusion (Kirchner et al, 2014). This study showed an increased use of RBC and platelets in the patients receiving PCC, however, this group had a significantly higher MELD score (P < 0·0001) than the group that did not receive coagulation factor concentrates.
Recommendations
- Prothrombin time (PT)/International Normalised Ratio (INR) does not reliably predict bleeding risk in patients with liver disease. Grade 1B.
- Heparinase tests are recommended at graft reperfusion to determine the extent of any heparin-like effect. Grade 2B.
- In bleeding patients, VHA (FIBTEM, TEG ff) should be used to guide fibrinogen replacement. Grade 1C.
- VHA can be used in liver transplant patients to reduce overall transfusion requirement (a normal VHA trace has a 95% negative predictive value for transfusion requirement). Grade 1C.
Cardiac surgery
The use of VHA has been studied in the setting of cardiac surgery. One publication compared all three VHAs in a prospective observational study of 35 patients, using them at 1- and 24-h post-operatively compared with SLTs, and found TEG to be the most sensitive in detecting abnormal platelet counts, prolonged aPTT and reduced fibrinogen (Espinosa et al, 2014). Sonoclot was the least sensitive, although none of the patients had markedly abnormal haemostasis and clinical outcomes were not assessed (Espinosa et al, 2014).
Prediction and diagnosis of bleeding/coagulopathy
The majority of studies looking at prediction of bleeding have been small and have used variable definitions of major bleeding, which makes comparison of studies difficult. Preoperative (pre-bypass) VHA testing as an isolated test has not been shown to predict bleeding. Post-bypass VHA testing is moderately predictive of bleeding, however most studies have shown that the values obtained are often still in the normal range, and that the difference between the pre- and post-bypass tests, rather than the absolute values obtained, predicts bleeding (Cherng et al, 1998; Davidson et al, 2008; Wasowicz et al, 2010; Lee et al, 2012; Sharma et al, 2014; Welsh et al, 2014). A change in any of the VHA variables >15% is most often associated with increased risk of bleeding.
Use of ROTEM/TEG/Sonoclot for guiding transfusion and haemostatic therapy
Viscoelastic haemostatic assays-directed management has been reported to improve overall clinical outcomes after cardiac surgery (Weber et al, 2012; Sartorius et al, 2014; Pearse et al, 2015; Trevisan et al, 2016), and result in less bleeding and lower need for re-exploration after coronary artery bypass grafting (CABG) (Speiss et al, 1995). Duration of hospitalisation was also reduced (Ichikawa et al, 2017). Conversely, a recent systematic review of 15 randomised trials involving 8737 patients found no significant difference in mortality, reoperation or postoperative recovery (Serraino & Murphy, 2017), with benefits being the reduction in transfusion requirements only. However, when used in combination with other techniques, such as a smaller bypass circuit (Mehaffey et al, 2017), or a general package of intervention (Ranucci et al, 2017) clinical outcomes have been significantly improved.
A TEG-guided approach can reduce use of allogeneic components by up to 58%, compared to SLTs (Westbrook et al, 2009). Similarly, a 30–80% reduction in blood component use has been demonstrated with intraoperative ROTEM (Royston & von Kier, 2001; Romlin et al, 2013; Karkouti et al, 2015; Ichikawa et al, 2017), and in the setting of hypothermic cardiac arrest for proximal cardiac surgery (Girdauskas et al, 2010; Fassl et al, 2013).
Ak et al (2009) showed that using the TEG parameters, R time and MA, to guide management led to a significant reduction in platelet and FFP use (n = 110) when compared to clinician-directed transfusion without TEG (n = 114). These results were replicated in a second study (Aoki et al, 2012). Systematic reviews of 9 (Fahrendorff et al, 2017) and 15 (Serraino & Murphy, 2017) studies where patients have been randomised to VHA-directed management or empirical management, have given differing results with respect to volume of platelet transfusions. However, the new generation VHAs incorporate platelet function testing and when specific cut-off values of preoperative platelet function tests have been used to direct timing of surgery, this has resulted in a significant reduction in surgical re-exploration rate, use of FFP and use of platelet concentrates (Ranucci et al, 2017).
In paediatric cardiac surgery, TEG-guided management has resulted in more effective cessation of postoperative bleeding compared to SLT-guided therapy (Niebler et al, 2012). Randomised trials have shown the same effect following ROTEM-directed therapy with significant reductions in postoperative bleeding and blood component requirements (Romlin et al, 2013; Nakayama et al, 2015). Analysis of ROC curves of ROTEM parameters in 150 children, showed EXTEM CT >111 s, EXTEM A10 <38 mm and FIBTEM A10 <3 mm could be used to guide management (Faraoni et al, 2015).
Studies have also found good correlation between FIBTEM and Clauss fibrinogen in adults (Ogawa et al, 2012a,b; Mace et al, 2016) and children (Pekelharan et al, 2014) and decreasing levels of fibrinogen can be quickly determined (Romlin et al, 2013). Ortmann et al (2015) showed superiority of FIBTEM over Clauss fibrinogen. VHA can be used as a valid assessment of fibrinogen concentration to guide fibrinogen replacement leading, in some cases, to complete avoidance of FFP and platelets (Rahe-Meyer et al, 2009).
In general, reduction in blood component use may be equal when guided by VHA or laboratory-derived algorithms but both appear to be better than clinician-directed transfusion (Avidan et al, 2004) and the delays in waiting for SLTs support the need for VHAs in the management of these patients.
Recommendations
- Pre-operative VHA has not been shown to be useful for predicting bleeding in patients having cardiac surgery. Grade 2B.
- Patients with a normal or abnormal postoperative VHA and no bleeding should not receive empirical blood components. Grade 2A.
- Superiority over laboratory tests in predicting bleeding has not been consistently demonstrated. Grade 2C.
- A single post-operative VHA test may not be useful for prediction of bleeding. However, if deterioration of VHA parameters is seen on repeat testing, the patient should be closely monitored for bleeding and intervention with appropriate blood components should be considered. Grade 2B.
- Cardiac surgery services should use transfusion protocols based on VHA testing to reduce use of blood components and potentially improve clinical outcomes in bleeding patients. Grade 2B.
- VHA can be used as a valid assessment of fibrinogen concentration to guide fibrinogen replacement. Grade 1B.
Trauma
Prediction of bleeding and coagulopathy
Many observational studies have explored whether VHA can reliably predict bleeding. Sixteen studies gave a threshold VHA parameter that could be used as a cut-off above/below which transfusion was more likely to be given, but very few gave data on the sensitivity/specificity of their reported VHA threshold. Broadly, the majority of the thresholds that predicted transfusion represented measurements of clot strength. Only one of these thresholds (EXTEM A5 35 mm, below which a patient is deemed to be at high risk of bleeding) has been externally validated (Davenport et al, 2011 – validated by Hagemo et al, 2015), limiting the validity of the other results to centres outside the reporting institution. Davenport et al (2011) demonstrated that an EXTEM A5 of 35 mm or more had a high NPV for transfusion need. The finding of VHA-detected fibrinolysis, commonly reported with TEG as >3% lysis, has also consistently been associated with patients who have required large transfusion volumes, however VHA are insensitive to mild/moderate fibrinolysis and should not be used to withhold TXA (Raza et al, 2013). Several systematic reviews have highlighted the limited nature of the available evidence (Da Luz et al, 2014; Whiting et al, 2015; Haas et al, 2014; Hunt et al, 2015b).
Diagnosis of traumatic coagulopathy/bleeding
Viscoelastic haemostatic assays tests have been used in a variety of ways to diagnose traumatic coagulopathy. A common method has been to explore the relationship between VHA and SLTs. Many studies report good correlations, with the majority defining coagulopathy using a PT-based measure. More trauma patients are found to have clotting abnormalities using ROTEM than using SLTs (64% vs. 10·5%)(Doran et al, 2010). No comparative data are available for TEG.
Viscoelastic haemostatic assays measures which are used to diagnose trauma coagulopathy vary but can be broadly summarised by three main changes: prolongation of clot formation, reduction in clot strength and increase in fibrinolysis. The most common abnormalities include: reduction in A and MCF (ROTEM) (Tauber et al, 2011; Wooley et al, 2013; Meyer et al, 2014) and reduction in MA (TEG) (Meyer et al, 2014; White et al, 2015) – all measures of clot strength, and low clot strength is viewed as an important marker, suggesting higher bleeding risk. However, no consensus value is yet agreed – for example Davenport et al (2011) used an EXTEM A5 of ≤35 mm as diagnostic of trauma coagulopathy whereas Hagemo et al (2015) used ≤40 mm. Other reported VHA changes include prolongation of CT and CFT and reduction of alpha angle (ROTEM) (Tauber et al, 2011), prolonged R times (TEG) (Meyer et al, 2014) and increased LY30 (rTEG) (Moore et al, 2017).
VHA for guiding transfusion and haemostatic therapy
Viscoelastic haemostatic assays-guided transfusion algorithms for management of severe trauma bleeding have been widely reported using TEG and ROTEM. TEG algorithms vary according to institution, although the general principles were similar across all studies. There are far fewer ROTEM data available and most studies arise from the same institution (Schochl et al, 2010, 2011; Innerhofer et al, 2017). These studies provide ROTEM values for transfusion of fibrinogen concentrate and PCC, with no clear guide for platelet transfusion and no mention of fibrinolysis.
The observational studies provide low quality data suggesting that VHA-guided transfusion algorithms (mostly TEG) reduce mortality (Johansson & Stensballe, 2009; Schochl et al, 2010; Kashuk et al, 2012; Tapia et al, 2013); change transfusion practices when compared to empiric therapy (Tapia et al, 2013; Mamczak et al, 2016) and reduce/avoid allogeneic transfusion (Schochl et al, 2011; Yin et al, 2014). Data from a pre- and post-implementation military study demonstrated increased use of blood components (platelets four-fold and cryoprecipitate two-fold) after incorporating ROTEM into resuscitation practices (Prat et al, 2017) despite similar patient characteristics and rates of coagulopathy. This study suggests that non VHA-based transfusion may underestimate the need for platelets and fibrinogen supplementation.
One RCT using rapid-TEG (Gonzalez et al, 2016) reported a significant reduction in death at 28 days with VHA: 20 deaths SLT (36·4%) vs. 11 VHA (19·6%). Median times to death were shorter in the VHA arm (4·2 h vs. 10·4 h) and numbers of haemorrhagic deaths lower (8·9% vs. 20%). This study provides evidence that VHA-guided transfusion may be beneficial for the management of acute bleeding in trauma, over and above the effects of the empiric 1:1:1 transfusion. However, the study's limitations must be highlighted: it was a single centre study; large volumes of crystalloids were used in resuscitation; participants were allocated to study arms according to the week of the year, and the VHA algorithm was changed after two-thirds of the participants were enrolled. It is impossible to say what effect this would have on outcome but raises questions about the overall validity of the study.
A second single-centre RCT used ROTEM-guided thresholds to compare FFP with factor concentrates (fibrinogen concentrate (FgC) and PCC) (Innerhofer et al, 2017). The trial used dual ROTEM measures (FIBTEM A10 >8 mm, EXTEM CT <78 s) in combination with a clinical measure of bleeding to define achievement of haemostasis with the primary endpoint of multiple organ failure. As a result of high treatment failure in the FFP arm (inability to correct coagulopathy and need for rescue therapy with PCC) the study was terminated early. The authors reported an association between clinically relevant bleeding and ROTEM measurements that could be used as a threshold to withhold transfusion. A large multi-centre RCT (iTACTIC) evaluating VHA- and SLT-based transfusion algorithms in trauma haemorrhage is due to be completed in 2018 and the results are awaited (NCT02593877).
Recommendations
- Normal VHA results confer a high negative predictive value for transfusion need, enabling the clinical team to monitor the patient closely without immediate activation of the major haemorrhage protocol. Grade 2B.
- Low clot strength measures on TEG and ROTEM and lysis of greater than 3% on TEG may be used as an indicator that a trauma patient is at higher risk of requiring RBC and blood components. Grade 2C.
- VHA, particularly TEG, may reduce mortality and reduce transfusion exposure and, if available, may be considered for transfusion guidance in trauma haemorrhage. Grade 2B.
- Tranexamic acid should not be withheld based on the TEG or ROTEM parameters. Grade 1B.
Practical use of VHA devices
The published literature focuses on the use of VHAs to enable early identification of coagulopathy and to guide transfusion of blood components. From a practical point of view, it is important to repeat VHA tests after transfusion to assess the effect of haemostatic treatment interventions and to determine that there is no further deterioration in the haemostatic profile in the context of ongoing bleeding. However, in the clinical setting, the confirmation of normal coagulation is one of the most valuable aspects of VHAs. Not only does this reduce unnecessary transfusion of blood components but it also directs clinical management to address the underlying cause of haemorrhage. This effect is not possible to quantify but is reinforced by clinicians practicing in units with access to VHAs.
General practice points for managing major haemorrhage using VHA
- Transfusion algorithms should be adapted according to local normal ranges and locally validated.
- Normal VHA parameters are a useful indicator that bleeding due to coagulopathy is unlikely and transfusion of blood components is unlikely to be needed.
- An abnormal VHA result is relatively poor at predicting patients who will bleed and changes in serial measurements may be more valuable.
- When blood component transfusion is necessary, the use of VHA to guide and monitor replacement has generally been found to reduce the volumes required and improve other measures of outcome.
How to approach a VHA trace – general principles for guiding transfusion therapy
There are no universally agreed algorithms however the general principles of how to approach VHA-guided transfusion are set out in Table 4.
|
||||
| Test result shows: | Clot initiation | Clot strength | Clot lysis | |
|---|---|---|---|---|
| Prolonged R, ACT or CT | Reduced MA/MCF, normal fibrinogenb | Reduced MA/MCF, low fibrinogenb | LY-30 >8% Li30 >15% | |
| What does this mean? |
Low clotting factors and/or low fibrinogen level Warfarin use Heparin use DOAC use (not Apixaban) |
Low platelets | Low fibrinogen | VHA detected fibrinolysisa |
| Therapy recommended |
FFP (PCC might be considered) |
Platelets | Cryoprecipitate or fibrinogen concentrate | Consider additional anti-fibrinolytica |
| Therapy groups | ||||
| Obstetric |
FFP if R or CT above the normal range. PCC is not recommended |
No data are available to guide platelet transfusion | Cryoprecipitate or fibrinogen concentrate if FIBTEM <7 mm or <12 mm in severe bleeding | No data are available for guiding antifibrinolytic therapy |
| Liver |
FPP if results above normal range (PCC might be considered) |
EXTEM MCF <35 mm | FIBTEM <7 mm |
Fibrinolysis at reperfusion may correct spontaneously. Anti-fibrinolytics are indicated in most other circumstances. |
| Cardiac | FFP if >15% above ULN | Platelets | Cryoprecipitate or FgC | |
| Trauma |
FFP if results ≥ULN PCC not recommended |
Give platelets if MA/MCF at lower end or below the normal range whilst ff or FIBTEM normal | Cryoprecipitate or FgC if FIBTEM or ff at lower end or below the normal range | TEG LY-30 ≥3% indicates clinically important lysis |
- The research in the top panel was originally published in Blood. Johansson et al (2014) © the American Society of Hematology.
- ACT, activated clotting time; CT, clotting time; DOAC, direct oral anticoagulant; ff, functional fibrinogen; FFP, fresh frozen plasma; FgC, fibrinogen concentrate; Li30, lysis index at 30 min; LY-30, lysis at 30 min; MA, maximal amplitude; MCF, maximum clot firmness; PCC, prothrombin complex concentrate; R, reaction time; TEG, thromboelastography; ULN, upper limit of normal; VHA, viscoelastic haemostatic assay.
- a Do not withhold an anti-fibrinolytic if is it clinically indicated and within 3 h of injury or start of postpartum haemorrhage.
- b Clot strength measures are highly dependent on platelets and fibrinogen. To differentiate low clot strength due to loss of platelet function or from that due to low fibrinogen levels, the clot strength of the fibrinogen assay (ff – TEG; FIBTEM – ROTEM) should be compared with the standard TEG or ROTEM EXTEM trace, respectively. A low overall clot strength with a normal ff/FIBTEM would suggest lack of platelets contributing to the clot, whereas a low overall clot strength with a concomitant low ff/FIBTEM would point to lack of fibrinogen.
Areas for future research
- Define measures for achievement of haemostasis to provide a consensus outcome for clinical study reporting, so that VHA results can be compared across studies and across patient groups
- Clinical studies to compare the efficacy and cost effectiveness of VHA-supported transfusion algorithms with standard care during obstetric and trauma bleeding
- Large multi-centre clinical trials evaluating TEG or ROTEM algorithms in major haemorrhage using standardised intervention points as well as standardised interventions, allowing comparison between studies
- Future studies are required to establish if improvement in the VHA profile equates to improvement in clinical outcome.
Conclusion
Viscoelastic haemostatic assays devices have practical advantages as point-of-care devices for monitoring major haemorrhage including speed of results and a set of parameters that assesses a global coagulation profile. However, the lack of a systematic approach to their use, with low quality published data that has not been clearly linked to important clinical outcomes means that, at present, the evidence base to guide practice is limited.
Acknowledgements
The authors wish to thank Carolyn Doree and the NHS Blood and Transplant Systematic Review Initiative for help in undertaking the literature search. The BSH Haemostasis and Thrombosis Task Force member was Professor Mike Laffan. The authors would like to thank him, the BSH sounding board, and the BSH guidelines committee for their support in preparing this guideline. All authors reviewed the literature search and contributed to the drafting and editing of the manuscript.
Declaration of interests
The BSH paid the expenses incurred during the writing of this guidance. All authors have made a declaration of interests to the BSH and Task Force Chairs which may be viewed on request. The following authors have the following declarations of interest: PC: research support from CSL Behring, Werfen and Haemonetics, paid consultancy from Werfen and CSL Behring, speaker fees from CSL Behring, educational grant from CSL Behring; SM: Haemonetics Scientific Advisory Council 2017; AK: AK's institution has received educational grant funding from Haemonetics and Hemosonics LLC for conducting studies using VHA; RD: paid consultancy for LFB, RD's institution has received reagent and equipment support from TEM; NC: paid consultancy for LFB, NC's institution has received reagent and equipment support from TEM. ML has received financial reimbursement (consultancy fees and speaker fees) from LFB, CSL, Behring, Pfizer and Shire. The remaining members of the writing group (SP, DK, HM) have no conflicts of interest to declare.
Review process
The document will be reviewed regularly by the relevant Task Force and the literature search will be re-run every 3 years to search for any RCT or other high-quality data that is either new or that may have been missed. The document will be archived and removed from the BSH current guidelines website if it becomes obsolete. If new recommendations are made an addendum will be published on the BSH guidelines website (www.b-s-h.org.uk).
Disclaimer
While the advice and information in this guidance is believed to be true and accurate at the time of going to press, neither the authors, the BSH nor the publishers accept any legal responsibility for the content of this guidance.




