Diagnostic markers for CNS lymphoma in blood and cerebrospinal fluid: a systematic review
Summary
Diagnosing central nervous system (CNS) lymphoma remains a challenge. Most patients have to undergo brain biopsy to obtain tissue for diagnosis, with associated risks of serious complications. Diagnostic markers in blood or cerebrospinal fluid (CSF) could facilitate early diagnosis with low complication rates. We performed a systematic literature search for studies on markers in blood or cerebrospinal fluid for the diagnosis CNS lymphoma and assessed the methodological quality of studies with the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2). We evaluated diagnostic value of the markers at a given threshold, as well as differences between mean or median levels in patients versus control groups. Twenty-five studies were included, reporting diagnostic value for 18 markers in CSF (microRNAs -21, -19b, and -92a, RNU2-1f, CXCL13, interleukins -6, -8, and -10, soluble interleukin-2-receptor, soluble CD19, soluble CD27, tumour necrosis factor-alfa, beta-2-microglobulin, antithrombin III, soluble transmembrane activator and calcium modulator and cyclophilin ligand interactor, soluble B cell maturation antigen, neopterin and osteopontin) and three markers in blood (microRNA-21 soluble CD27, and beta-2-microglobulin). All studies were at considerable risk of bias and there were concerns regarding the applicability of 15 studies. CXCL-13, beta-2-microglobulin and neopterin have the highest potential in diagnosing CNS lymphoma, but further study is still needed before they can be used in clinical practice.
Diagnostic delay is still a major problem in central nervous system (CNS) lymphoma (Haldorsen et al, 2005). Although empirical evidence for the advantages of early diagnosis (for survival or other outcome measures) is lacking, the rapidly progressive nature of the disease and the – potentially irreversible – consequences of progressive CNS symptoms have led to the recommendation to obtain diagnosis as rapidly as possible (Nayak et al, 2015).
Primary CNS lymphomas (PCNSLs) account for 2·2% of primary CNS tumours (Dolecek et al, 2012). The majority (95%) of PCNSLs are histologically classified as a type of diffuse large B cell lymphoma (DLBCL) and the remaining are diverse such as Burkitt, lymphoblastic, marginal zone or T cell lymphomas (Ferreri & Marturano, 2012). The highest rate of secondary CNS lymphoma (SCNSL) involvement (27%) is found in lymphoblastic lymphomas and Burkitt lymphomas (van de Schans et al, 2012). Intensive treatment with corticosteroids, chemotherapy and radiotherapy may be curative or offer effective (and sometimes long-lasting) palliation in patients with PCNSL who are in fair to good clinical condition (Morris & Abrey, 2009). Tumour resection through neurosurgery does not result in therapeutic benefit; hence, only biopsy is indicated to obtain a tissue diagnosis (Bellinzona et al, 2005). Clinical and radiological features may suggest the diagnosis of PCNSL, but are not diagnostic; in a typical clinical situation, a wide differential diagnosis will be considered, including other malignancies, demyelinating disease (multiple sclerosis) and neurosarcoidosis (Küker et al, 2005; Zacharia et al, 2008; Ricard et al, 2012). Secondary CNS lymphoma typically presents with leptomeningeal, subependymal or cranial nerve contrast enhancement, which can also be seen in infections or inflammatory disease (Haldorsen et al, 2011).
Brain biopsy is the gold standard for the diagnosis of CNS lymphoma. This invasive procedure has a complication rate of 8·5%, consisting of haematomas, seizures or brain oedema, and biopsy-related mortality occurs in 1% (Khatab et al, 2014). Less invasive diagnostic options are cytology or flow cytometry of cerebrospinal fluid (CSF) or vitreous fluid (Scott et al, 2013). However, these are only positive in case of leptomeningeal or ocular involvement respectively. Also, the finding of a monoclonal population of lymphocytes may rarely be a false-positive result, because other diseases may also cause such a response (Vafaii & DiGiuseppe, 2014). Often steroid therapy is initiated to reduce symptoms before pathological diagnosis is obtained. The effects of corticosteroids, however, also make it more difficult to obtain a definite (histopathological) diagnosis, with an increased chance of false-negative findings (Roth et al, 2010).
Therefore, there is need for a diagnostic test with a high diagnostic yield and limited risks, leading to shorter time to diagnosis. A diagnostic marker obtainable from blood or CSF would fulfil this need, serving as a type of ‘liquid biopsy’. For that reason, we performed a systematic and critical review of the literature on possible diagnostic markers for the diagnosis of central nervous system lymphoma.
Methods
We performed a systematic literature search in PubMed using synonyms for (i) CNS lymphoma, (ii) blood or CSF, and (iii) markers. Articles were selected for title, abstract and full text, based on the inclusion and exclusion criteria presented in Fig 1. There were insufficient data for a quantitative meta-analysis of any given diagnostic marker and therefore a systematic descriptive analysis of the available data was performed. This review was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al, 2009).
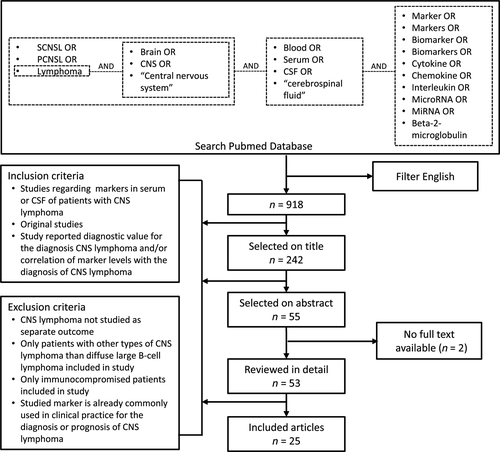
We assessed the quality of all included studies using the 2011 revised version of ‘Quality Assessment of Diagnostic Accuracy Studies’ (QUADAS-2) (Whiting et al, 2011). The QUADAS-2 is designed for grading individual diagnostic studies on their risk of bias and applicability. As recommended in QUADAS-2, we adapted signalling questions to our review question. We omitted the signalling questions about blinding of the reference and index test, because the results of these tests are not at great risk for bias; the results consist of quantitative or dichotomous outcomes with little room for interpretation. Concerns regarding applicability were scored ‘high’ when the control group did not consist of patients with a clinical picture suggestive of CNS lymphoma, as this does not correspond to our review question. On the basis of the quality assessment of the included studies, we determined the risk of bias and applicability of each individual biomarker. When classifications of risk of bias or applicability differed among different studies, we used the risk assessment of the most important study on this biomarker, based on sample size and quality of the study.
Results
The search query in PubMed yielded 918 results, of which 25 studies were included (Fig 1). These studies showed a diagnostic value for levels of certain markers in blood or CSF in the diagnosis of primary or secondary CNS lymphoma. Studies that did not investigate diagnostic value but did find significant differences in levels of a marker between patient (CNS lymphoma) and control groups, were also included. The included studies concern the following markers: micro RNA (miR)-21, miR-19b, miR92a, U2 small nuclear RNA fragments (RNU2-1f), C-X-C motif chemokine Ligand 13 (CXCL13), interleukin (IL)-6, IL-8, IL-10, soluble IL-2 receptor (sIL-2R), soluble CD19 (sCD19), soluble CD27 (sCD27), tumour necrosis factor alfa (TNF-α), beta-2-microglobulin (B2M), antithrombin III (ATIII), soluble transmembrane activator and calcium modulator and cyclophilin ligand interactor (sTACI), soluble B cell maturation antigen (sBCMA), neopterin and osteopontin.
Markers
The diagnostic values of markers, as derived from the included studies, are listed for PCNSL (Table 1) (Kersten et al, 1996; Murase et al, 2000; Roy et al, 2008; Baraniskin et al, 2011, 2012a, 2016; Sasayama et al, 2012; Rubenstein et al, 2013; Mao et al, 2014; Kuusisto et al, 2015; Sasagawa et al, 2015; Viaccoz et al, 2015; Mabray et al, 2016; Nguyen-Them et al, 2016; Song et al, 2016; Strehlow et al, 2016; Ikeguchi et al, 2017; Thaler et al, 2017) and for SCNSL (Table 2) (Ernerudh et al, 1987; Kersten et al, 1996; Kara et al, 2007; Muñiz et al, 2014). Some of the included articles reported the diagnostic values for PCNSL and SCNSL as one patient group (Roy et al, 2008), these results are also included in Table 1. Mean or median levels of markers in CNS lymphoma versus control patients are presented in Table 3. Details of patient and control group of each study can be found in supplementary Table 1. The following paragraphs summarise the results for each marker.
| Study | Retrospective or prospective | Patient group | Control group | Marker | Serum or CSF | Cut-off | AUC [95% CI] | Sens (%) | Spec (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis (n) | N | Diagnosis (n) | N | ||||||||
| Ikeguchi et al (2017) | Retrospective | PCNSL (8) and SCNSL (4) | 12 | Multiple Sclerosis (64), NMOSD (35), TDL (17) | 116 | sIL-2R | CSF | 0·87 | 83·3 | 90·0 | |
| IL-10 | CSF | ||||||||||
| Thaler et al (2017) | Prospective | PCNSLa | 33 | Primary brain tumour (20), secondary brain tumour (22), neuroinfectious disease (13), neuroinflammatory disease (30), other neurological diseases (52) | 137 | sTACI | CSF | >68·4 pg/ml | 0·94 [0·88–0·99] | 87·9 | 88·3 |
| sBCMA | CSF | >460 pg/ml | 0·76 [0·66–0·86] | 72·7 | 71·8 | ||||||
| sTACI + sBCMA | CSF | >68·4 pg/ml; >460 pg/ml | 63·9 | 96·7 | |||||||
| Baraniskin et al (2016) | Retrospective | PCNSL | 72 | CNS inflammation (35), miscellaneous neurological disorders (12) | 47 | RNU2-1f | CSF | >5·7 REL | 0·91 | 68·1 | 91·4 |
| miR-21 | CSF | >7·3 REL | 0·99 | 91·7 | 95·7 | ||||||
| Mabray et al (2016) | Retrospective | PCNSL (38) and SCNSL (5) | 43 | Metastases (21), high-grade gliomas (14), tumefactive demyelinating lesions (9) | 44 | CXCL-13 | CSF | >103·0 pg/ml | 0·83 [0·74–0·90] | 76·7 | 90·9 |
| IL-10 | CSF | >21·77 pg/ml | 0·79 [0·69–0·87] | 62·8 | 95·5 | ||||||
| Nguyen-Them et al (2016) | Prospective | PCNSL | 112 | Glioma (9), ependymoma (2), medulloblastoma (2) brain metastasis (3), neuroinflam-matory disease (18), neuroinfectious disease (3), neurodegenerative disease (3) | 40 | IL-10 | CSF | >4 pg/ml | 0·88 | 88·6 | 88·9 |
| Song et al (2016) | Retrospective | PCNSL | 22 | Systemic NHL with high risk of CNS involvement (41), CNS inflammatory disease (14), CNS infection (13), CNS demyelinating disease (5), other brain tumours (7) | 80 | IL-10 | CSF | >8·2 pg/ml | 0·96 [0·90–1·00] | 95·5 | 96·1 |
| IL-6 | CSF | >5·1 pg/ml | 0·61 [0·48–0·74] | 54·6 | 70·1 | ||||||
| IL-8 | CSF | >117 pg/ml | 0·56 [0·42–0·69] | 31·8 | 83·1 | ||||||
| TNF-α | CSF | >5·3 pg/ml | 0·66 [0·41–0·68] | 59·1 | 57·1 | ||||||
| IL-10/IL-6 ratio | CSF | >0·72 | 0·98 [0·93–1·00] | 95·6 | 100 | ||||||
| Strehlow et al (2016) | Retrospective | PCNSL (29) and SCNSL (8) | 37 | Inflammatory CNS disease (6), Multiple sclerosis (8), glioblastoma (9), healthy controls (13) | 36 | Osteopontin | CSF | >400 ng/ml | 0·93 [0·87–1·00] | 87 | 86 |
| Kuusisto et al (2015) | Retrospective | PCNSL (5), SCNSL (7) and systemic lymphoma (29) | 41 | Alzheimer disease (19), Multiple Sclerosis (6), no neurological disease (17) | 42 | Antithrombin III | CSF | 0·79 | |||
| Albumin | CSF | 0·84 | |||||||||
| AT III/albumin ratio | CSF | 0·25 | |||||||||
| Sasagawa et al (2015) | Retrospective | PCNSL (15) and SCNSL (4) | 19 | Glioblastoma (5), anaplastic astrocytoma (2), glioma (1), ependymoma (1), metastasis (5), immature teratoma (1), atypical meningioma (1), undifferentiated sarcoma (1), Multiple Sclerosis (3), other (6) | 26 | IL-10 | CSF | >3 pg/ml | 0·97 | 94·7 | 100 |
| IL-10/IL-6 ratio | CSF | >2·2 | 0·95 | 68·4 | 96·1 | ||||||
| sIL-2R | CSF | >60·4 u/ml | 0·91 | 94·7 | 84·6 | ||||||
| Β2M | CSF | >2·4 mg/l | 0·81 | 89·4 | 88·5 | ||||||
| Viaccoz et al (2015) | Retrospective | PCNSL | 28 | Other space occupying brain lesions (glioma (36), metastasis (13), other type of brain tumour (5), pseudotumoural inflam-matory brain lesion (13)), nontumefactive inflammatory CNS disorders (29) | 96 | Neopterin | CSF | >10 nmol/l | 96 | 92 | |
| Mao et al (2014) | Retrospective | PCNSL | 56 | Other CNS malignancy (55), CNS inflammation (20), healthy (47) | 122 | miR-21 | Serum | 0·93 [0·88–0·98] | |||
| Glioblastoma | 32 | 0·88 [0·81–0·95] | |||||||||
| Rubenstein et al (2013) | Retrospective | PCNSL (60) and SCNSL (23) | 83 | Neuro-inflammation (71), primary brain tumour (8), brain metastasis (12), neoplasm/infection outside CNS (46) | 137 | CXCL13 | CSF | >90 pg/ml | 69·9 | 92·7 | |
| IL-10 | CSF | >16·15 pg/ml | 65·4 | 92·6 | |||||||
| CXCL13 and IL-10 | CSF | >90 pg/ml; >16 pg/ml | 83 | 87 | |||||||
| CXCL13 and/or IL-10 | CSF | >90 pg/ml; >16 pg/ml | 99 | ||||||||
| PCNSL HIV negative | 55 | Neuro-inflammation (71), primary brain tumour (8), brain metastasis (12), neoplasm/infection outside CNS (46) | 137 | CXCL13 | CSF | >116 pg/ml | 0·84 [0·78–0·89] | 71 | 95 | ||
| IL-10 | CSF | >23 pg/ml | 0·83 [0·79–0·90] | 64 | 94 | ||||||
| CXCL13 or IL-10 | CSF | >116 pg/ml; >23 pg/ml | 0·87 [0·82–0·92] | 84·2 | 90·5 | ||||||
| CXCL13 and IL-10 | CSF | >116 pg/ml; >23 pg/ml | 0·75 [0·68–0·81] | 50 | 99·3 | ||||||
| Baraniskin et al (2012a) | Retrospective | PCNSL | 39 | CNS inflammation, other | 37 | miR-21 and miR-19b and/or miR-92a | CSF | >8·0 REL; >1·4 REL; >2·5 REL; | 97·4 | ||
| Sasayama et al (2012) | Retrospective + prospective part | PCNSL | 31 | Other CNS malignancy (57), CNS inflammation (2) | 59 | IL-10 | CSF | 9·5 pg/ml | 0·92 [0·84–0·99] | 71 | 100 |
| IL-6 | CSF | 4·0 pg/ml | 0·68 [0·57–0·79] | 77 | 63 | ||||||
| B2M | CSF | 2056 μg/l | 0·93 [0·87–1·00] | 88 | 90·3 | ||||||
| sIL-2R | CSF | 77 u/ml | 0·85 [0·75–0·96] | 81 | 56·7 | ||||||
| Baraniskin et al (2011) | Retrospective | PCNSL | 23 | CNS inflammation (20), other (10) | 30 | miR-21 | CSF | >8·0 REL | 0·94 [0·87–1·00] | 95·7 | 83·3 |
| miR-19b | CSF | >1·4 REL | 0·98 [0·91–1·00] | 95·7 | 87·7 | ||||||
| miR-92a | CSF | >2·5 REL | 0·97 [0·93–1·00] | 95·7 | 80 | ||||||
| miR-21 and miR-19b and/or miR-92a | CSF | >8·0 REL; >1·4 REL; >2·5 REL | 95·7 | 96·7 | |||||||
| Roy et al (2008) | Retrospective | PCNSL and SCNSLb | 24 | Malignancy outside CNS (29), CNS inflammation (15), other (33) | 77 | ATIII | CSF | 1·2 μg/l | 0·91 | 75 | 98·7 |
| Murase et al (1998) | Retrospective | PCNSL | 12 | Other CNS malignancy | 30 | sCD27 | CSF | 15 U/ml | 100 | 100 | |
| Kersten et al (1996) | Retrospective | PCNSLc | 7 | Malignancy outside CNS, other CNS malignancy, PCNSL in remission (1) | 41 | sCD27 | CSF | >10 U/ml | 100 | 98 | |
- PCNSL, primary central nervous system lymphoma; SCNSL, secondary central nervous system lymphoma; NMOSD, neuromyelitis optica spectrum disorder; TDL, tumefactive demyelinating lesions; NHL, non-Hodgkin lymphoma; AUC, area under the curve; CI, confidence interval; sens, sensitivity; spec, specificity; REL, expression of miRNA relative to miR-24. For the investigated markers, abbreviations are used; see main text for their full names.
- a Patient group included 6 SCNSL patients, however diagnostic value only examined for PCNSL patients.
- b Numbers not specified.
- c These are 8 samples form 4 PCNSL patients (one sample after treatment, when PCNSL was in remission) and 40 samples from 32 patients with other CNS malignancies (13) or malignancy outside CNS (19).
| Study | Retropective or prospective | Patient group | Control group | Marker | Serum or CSF | Cut-off | AUC; P-value | Sens | Spec | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis (n) | N | Diagnosis (n) | N | ||||||||
| Muñiz et al (2014) | Retrospective | SCNSL | (40)a | DLBCL and BL without CNS involvement | (40)a | CD19 | CSF | >1·18 ng/ml | 0·88 P < 0·05 | 80% | 95% |
| B2M | CSF | >2·56 ng/ml | 0·78 P < 0·05 | 62% | 90% | ||||||
| Kara et al (2007) | Retrospective | CNS localization of ALL (3), NHL (1), AML (1) | 5 | ALL (15), NHL (6), AML (4), epidural haemorrhage (2), lumbar disc protrusion (3) | 30 | sCD27 | CSF | >350 u/ml | |||
| Kersten et al (1996) | Retrospective | SCNSL/CNS localization of ALL | (104)b | NHL and ALL without CNS involvement (children) | (104)b | sCD27 | CSF | >10 u/ml | 0·99 | 83% | 86% |
| SCNSL/CNS localization of ALL | (70)c | NHL and ALL without CNS involvement | (70)c | sCD27 | CSF | >10 u/ml; | 0·95 | 100% | 82% | ||
| SCNSL/CNS localization of ALL | (38)c | NHL and ALL without CNS involvement | (38)c | sCD27 CSF/serum ratio | CSF/serum | >0·06 | 0·91 | 83% | 88% | ||
| SCNSL/CNS localization of ALL | (54)c | NHL and ALL without CNS involvement | (54)c | B2M | CSF | >1·6 mg/l | 0·77 | 81% | 68% | ||
| SCNSL/CNS localization of ALL | (36)c | NHL and ALL without CNS involvement | (36)c | B2M CSF/serum ratio | CSF/serum | >0·7 | 0·82 | 92% | 63% | ||
| Ernerudh et al (1987) | Retrospective | SCNSL | 14 | NHL and HD without CNS involvement | 12 | B2M | CSF | >1·9 mg/l | 71% | 67% | |
- SCNSL, secondary central nervous system lymphoma; DBLCL, diffuse large B cell lymphoma; BL, Burkitt lymphoma; NHL, non-Hodgkin lymphoma; ALL, acute lymphatic leukemia; AUC, area under the curve; sens, sensitivity; spec, specificity.
- a Number of patients in patients group plus control group.
- b Data are based on 104 samples of 70 children with ALL/NHL undergoing routine CNS staging.
- c Data are based on 70 samples of 45 patients with ALL/NHL suspected of meningeal localization, sCD27 CSF/serum ratio, B2M and B2M CSF/serum ratio were only measured in a part of these samples.
| Study | Retro- or prospective | Marker | Serum or CSF | Patient group | Control group | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | N | Mean ± SD or median (range) | Diagnosis (n) | N | Mean ± SD or median (range) | |||||
| Ikeguchi et al (2017) | Retrospective | sIL-2R | CSF | PCNSL + SCNSL | 12 | 120 U/ml (71–330 U/ml) | Multiple Sclerosis (64), NMOSD (35), TDL (17), glioma (10) | 126 | <54·5 U/ml | P < 0·001 |
| IL-10 | CSF | 5·5 pg/ml (≤2·0–13·0 pg/ml) | ≤2·0 pg/ml | P < 0·01 | ||||||
| Thaler et al (2017) | Prospective | sTACI | CSF | PCNSL | 33 | 445 pg/ml (IQR 1883 pg/ml) | Primary brain tumour (20), secondary brain tumour (22), neuroinfectious disease (13), neuroinflammatory diseases (30), other neurological diseases (52) | 137 | 0 pg/ml (IQR 7 pg/ml) | |
| SCNSL | 6 | 32 pg/ml (IQR 3583 pg/ml) | ||||||||
| Serum | PCNSL | 21 | 348 pg/ml (IQR 289 pg/ml) | 338 pg/ml (IQR 291 pg/ml) | NS | |||||
| sBCMA | CSF | PCNSL | 33 | 760 pg/ml (IQR 1740 pg/ml) | 290 pg/ml (IQR 320 pg/ml) | |||||
| SCNSL | 6 | 430 pg/ml (IQR 3095 pg/ml) | ||||||||
| Serum | PCNSL | 21 | 15·4 ng/ml (IQR 11·2 ng/ml) | 15·5 ng/ml (IQR 6·4 ng/ml) | NS | |||||
| Baraniskin et al (2016) | Retrospective | RNU2-1f | CSF | PCNSL | 72 | 24·78 ± 0·32 Ct values | CNS inflammation (35), miscellaneous neurological disorders (12) | 47 | 29·07 ± 0·33Ct values | P < 0·0001 |
| Serum | 22·01 ± 0·43Ct values | 21·34 ± 0·64 Ct values | NS | |||||||
| Mabray et al (2016) | Retrospective | CXCL-13 | CSF | PCNSL + SCNSL | 43 | 2960·49 pg/ml (95% CI 1124·96–4796·01 pg/ml) | Metastases (21), high-grade gliomas (14), tumefactive demyelinating lesions (9) | 44 | 72·54 pg/ml (95% CI 9·02–136·05 pg/ml) | P < 0·001 |
| IL-10 | CSF | 557·48 pg/ml (95% CI 167·49–947·47 pg/ml) | 5·93 pg/ml (95% CI 3·42–8·43) | P < 0·001 | ||||||
| Nguyen-Them et al (2016) | Prospective | IL-10 | CSF | PCNSLa | 112 | |||||
| Song et al (2016) | Retrospective | IL-10 | CSF | PCNSL | 22 | 74·7 pg/ml (<5·0–1000 pg/ml) | High-risk systemic NHL | 41 | <5·0 pg/ml (<5·0–197·0 pg/ml) | P < 0·0001 PCNSL vs. all others |
| CNS inflammatory disease | 14 | <5·0 pg/ml | ||||||||
| CNS demyelinating disease | 5 | <5·0 pg/ml | ||||||||
| CNS infection | 13 | <5·0 pgml (<5·0–231·0 pg/ml) | ||||||||
| Other brain tumours | 7 | <5·0 pg/ml (<5·0–7·9 pg/ml) | ||||||||
| IL-6 | CSF | PCNSL | 22 | 5·2 pg/ml (<2·0–109·0 pg/ml) | High-risk systemic NHL | 41 | 2·5 pg/ml (<2·0–8·2 pg/ml) | P < 0·01 PCNSL vs. high-risk systemic NHL | ||
| CNS inflammatory disease | 14 | 3·2 pg/ml (<2·0–17·8 pg/ml) | ||||||||
| CNS demyelinating disease | 5 | 2·2 pg/ml (<2·0–3·9 pg/ml) | ||||||||
| CNS infection | 13 | 271·0 pg/ml (<2·0–1000 pg/ml) | ||||||||
| Other brain tumours | 7 | 7·3 pg/ml (6·4–70·8 pg/ml) | ||||||||
| IL-8 | CSF | PCNSL | 22 | 89·5 pg/ml (25·0–208·0 pg/ml) | High-risk systemic NHL | 41 | 61·5 pg/ml (28·0–129 pg/ml) | |||
| CNS inflammatory disease | 14 | 78·0 pg/ml (43·0–116·0 pg/ml) | ||||||||
| CNS demyelinating disease | 5 | 39·0 pg/ml (35·0–50·0 pg/ml) | ||||||||
| CNS infection | 13 | 232·0 pg/ml (22·0–7500 pg/ml) | ||||||||
| Other brain tumours | 7 | 96·0 pg/ml (17·0–312·0 pg/ml) | ||||||||
| TNF-α | CSF | PCNSL | 22 | 5·7 pg/ml (4·5–10·3 pg/ml) | High-risk systemic NHL | 41 | 5·0 pg/ml (<4·0–7·1 pg/ml) | |||
| CNS inflammatory disease | 14 | 5·7 pg/ml (4·8–6·7 pg/ml) | ||||||||
| CNS demyelinating disease | 5 | 4·1 pg/ml (<4·0–5·0 pg/ml) | ||||||||
| CNS infection | 13 | 5·8 pg/ml (4·3–50·1 pg/ml) | ||||||||
| Other brain tumours | 7 | 4·8 pg/ml (4·2–15·1 pg/ml) | ||||||||
| IL-10/IL-6 ratio | CSF | PCNSL | 22 | 12·6 (0·00–114·9) | High-risk systemic NHL | 41 | 0·0 (0·0–40·2) | P < 0·0001 PCNSL vs. all other diseases except other brain tumours | ||
| CNS inflammatory disease | 14 | 0·0 (0·0–0·0) | ||||||||
| CNS demyelinating disease | 5 | 0·0 (0·0–0·0) | ||||||||
| CNS infection | 13 | 0·0 (0·0–0·2) | ||||||||
| Other brain tumours | 7 | 0·0 (0·0–0·1) | ||||||||
| Strehlow et al (2016) | Retrospective | Osteopontin | CSF | PCNSL +SCNSL | 37 | 620 ng/ml (60–890 ng/ml) | Inflammatory CNS disease | 6 | 356 ng/ml (253–531 ng/ml) | P < 0·05 |
| Multiple sclerosis | 8 | 163 ng/ml (29–370 ng/ml) | P < 0·01 | |||||||
| Glioblastoma | 9 | 41 ng/ml (22–191 ng/ml) | P < 0·01 | |||||||
| Healthy controls | 13 | 319 ng/ml (142–430 ng/ml) | P < 0·01 | |||||||
| Serum | 52 ng/ml (17–231 ng/ml) | Inflammatory CNS disease | 6 | 99 ng/ml (40–273 ng/ml) | P = 0·158 PCNSL vs. inflammatory CNS disease and healthy controls | |||||
| Multiple sclerosis | 8 | |||||||||
| Glioblastoma | 9 | |||||||||
| Healthy controls | 13 | 59 ng/ml (22–203 ng/ml) | ||||||||
| Kuusisto et al (2015) | Retrospective | AT III | CSF | PCNSL + SCNSL + systemic lymphoma | 41 | 1·47 μg/ml (0·49–6·91 μg/ml) | Alzheimer's (19), Multiple Sclerosis (6), no neurological disease (17) | 42 | 1·08 μg/ml (0·50–4·05 μg/ml) | P = 0·002 |
| Sasagawa et al (2015) | Retrospective | IL-10 | CSF | PCNSL + SCNSL | 19 | 28 pg/ml (≤2–4100 pg/ml) | Gliobastoma (5), anaplastic astrocytoma (2), ependymoma (1), glioma (1), metastasis (5), immature teratoma (1), atypical meningioma (1), undifferentiated sarcoma (1), other (9) | 26 | <2·0 pg/ml | |
| IL-6 | CSF | 10·8 pg/ml (1·2–127 pg/ml) | 3·9 pg/ml (0·4–1790 pg/ml) | |||||||
| sIL-2R | CSF | 225 U/ml (<54·5–2750 U/ml) | <54·5 U/ml (<54·5–152 U/ml) | |||||||
| B2M | CSF | 3·9 mg/l (1·7–11·8 mg/l) | 1·5 mg/l (0·3–6·6 mg/l)) | |||||||
| Viaccoz et al (2015) | Retrospective | Neopterin | CSF | PCNSL | 28 | 41·8 nmol/l (7·70–135·0 nmol/l) | Glioma (36), metastasis (13), other type of brain tumour (5) | 54 | 5·1 nmol/l (1·90–16·90 nmol/l) | PCNSL vs. tumoural and pseudotumoural groups: P < 0·001 |
| Pseudotumoural inflammatory brain lesion | 13 | 4·3 nmol/l (1·9–29·2 nmol/l) | ||||||||
| Nontumefactive inflammatory CNS disorders | 29 | 3·8 nmol/l (1·7–26·5 nmol/l) | PCNSL vs. nontumefactive disorders: P < 0·001 | |||||||
| Kitai et al (2013) | Retrospective | sIL-2R | Serum | PCNSL | 13 | 629·5 ± 586·0 U/ml | Other CNS malignancy (14), CNS inflammation (3), other (7) | 24 | 408·5 ± 250·7 U/ml | NS |
| sIL-2R | Serum | PCNSLb | 12 | 646·0 ± 609·1 U/ml | Other CNS malignancy (11), CNS inflammation (3), other (5)b | 19 | 347·0 ± 166·6 U/ml | P < 0·025 | ||
| Rubenstein et al (2013) | Retrospective | CXCL13 | CSF | PCNSL new diagnosis | 43 | 5926·2 ± 2030 pg/ml | Neuro-inflammation | 71 | 44·9 ± 19 pg/ml | Recurrent CNS lymphoma vs. new diagnosis: P < 0·0005 new diagnosis CNS lymphoma vs. all controls: P < 1 × 10−7 |
| CXCL13 | CSF | SCNSL new diagnosis | 10 | 1783 ± 896 pg/ml | Primary brain tumour | 8 | 84·5 ± 60·7 pg/ml | |||
| CXCL13 | CSF | PCNSL recurrent | 17 | 996 ± 312 pg/ml | Brain metastasis | 12 | 58·46 ± 41·6 pg/ml | |||
| CXCL13 | CSF | SCNSL recurrent | 13 | 539 ± 157 pg/ml | Neoplasm/infection outside CNS | 46 | 11 ± 3·6 pg/ml | |||
| IL-10 | CSF | PCNSL new diagnosis | 43 | 282·9 ± 113 pg/ml | Neuro-inflammation | 71 | 5·6 ± 2·3 pg/ml | All CNS lymphoma vs. all controls P < 2·3 × 10−5 | ||
| IL-10 | CSF | SCNSL new diagnosis | 10 | 57 ± 37 pg/ml | Primary brain tumour | 8 | 10·9 ± 5·6 pg/ml | |||
| IL-10 | CSF | PCNSL recurrent | 17 | 1663 ± 483 pg/ml | Brain metastasis | 12 | 5·3 ± 1·5 pg/ml | |||
| IL-10 | CSF | SCNSL recurrent | 13 | 302 ± 126 pg/ml | Neoplasm/infection outside CNS | 46 | 3·6 ± 1·4 pg/ml | |||
| Baraniskin et al (2012a) | Retrospective | miR-21 | Serum | PCNSL | 14 | 22·60 ± 0·12 Ct values | Other | 8 | 22·63 ± 0·58 Ct values | NS |
| Sasayama et al (2012) | Retrospective | IL-10 | CSF | PCNSL | 26 | 27 pg/ml (<2–1610 pg/ml) | Other CNS malignancy | 40 | All <2·0 pg/ml | P < 0·001 |
| IL-6 | CSF | PCNSL | 26 | 5·7 pg/ml (1·2–264 pg/ml) | Other CNS malignancy | 40 | 2·7 pg/ml (0·8–478 pg/ml) | P = 0·017 | ||
| B2M | CSF | PCNSL | 26 | 4084 μg/l (970–11239 μg/l) | Other CNS malignancy | 40 | 1200 μg/l (172–2600 μg/l) | P < 0·001 | ||
| sIL-2R | CSF | PCNSL | 26 | 100 U/ml (<50–978 U/ml) | Other CNS malignancy | 40 | <50 U/ml (<50–87 U/l) | P < 0·001 | ||
| Prospective | IL-10 | CSF | PCNSL | 5 | 42 pg/ml (5–118 pg/ml) | Other CNS malignancy (17), CNS inflammation | 19 | <2·0 pg/ml (2·0–9·0 pg/ml) | P < 0·001 | |
| IL-6 | CSF | PCNSL | 5 | 6·8 pg/ml (4·4–13·9 pg/ml) | Other CNS malignancy (17), CNS inflammation | 19 | 2·7 pg/ml (0·8–221 pg/ml) | P = 0·017 | ||
| B2M | CSF | PCNSL | 5 | 4983 μg/l (2303–6166 μg/l) | Other CNS malignancy (17), CNS inflammation | 19 | 1164 μg/l (410–2075 μg/l) | P < 0·001 | ||
| sIL-2R | CSF | PCNSL | 5 | 88 U/ml (<50–3495 U/ml) | Other CNS malignancy (17), CNS inflammation | 19 | All <50 U/ml | P < 0·001 | ||
| Baraniskin et al (2011) | Retrospective | miR-21 | CSF | PCNSL | 23 | 23·5 REL (7·9–291·7 REL) | CNS inflammation (20), other (10) | 30 | 1·65 REL (0–23·8 REL) | |
| miR-19b | CSF | PCNSL | 23 | 6·8 REL (1·4–94·9 REL) | CNS inflammation (20), other (10) | 30 | 0·25 REL (0–5·1 REL) | |||
| miR-92a | CSF | PCNSL | 23 | 12·8 REL (2·6–128·8 REL) | CNS inflammation (20), other (10) | 30 | 0·9 REL (0·2–18 REL) | |||
| Fischer et al (2009) | Retrospective | CXCL13 | CSF | PCNSL and SCNSL | 29 | 468 pg/ml (41–1384 pg/ml) | Other CNS malignancy | 9 | 6 pg/ml (0–601 pg/ml) | P = 0·0005 |
| Malignancy outside CNS (9), other (20)c | 25 | 8 pg/ml (0–187 pg/ml) | P < 0·0001 | |||||||
| Roy et al (2008) | Retrospective | ATIII | CSF | PCNSL and SCNSL | 24 | 1·68 μg/ld | Malignancy outside CNS (29), CNS inflammation (15), other (33) | 77 | 0·54 μg/ld | P < 3·3 × 10−9, when normalized for total CSF protein P < 0·015 |
| Brain metastasis | 13 | 2·6 μg/ld | ||||||||
| Kara et al (2007) | Retrospective | sCD27 | CSF | CNS localization of ALL, AML, NHL | 5 | 141·5 pg/ml (31·43–459·60 pg/ml) | ALL (15), NHL (6), AML (4), epidural hemorrhage (2), lumbar disc protrusion (3) | 30 | ||
| Salmaggi et al (2000) | Retrospective | IL-10 | CSF | PCNSL | 11 | 14·8 ± 23 pg/ml | Other CNS malignancy | 11 | 0·23 ± 0·26 pg/ml | P < 0·01 |
| Murase et al (1998) | Retrospective | sCD27 | CSF | PCNSL | 12 | 84·5 ± 133 U/ml | Other CNS malignancy | 30 | 4·6 ± 3·2 U/ml | P < 0·001 |
| Ernerudh et al (1987) | Retrospective | B2M | CSF | SCNSL | 13 | 3·9 ± 3·4 mg/l | NHL without CNS involvement | 11 | 1·7 ± 1·0 mg/l | P < 0·05 |
| Mavligit et al (1980) | Retrospective | B2M | CSF | SCNSL | 6 | 4·2 ± 0·6 mg/l | NHL without CNS involvement | 14 | 1·9 ± 0·3 mg/l | P < 0·02 |
- PCNSL, primary central nervous system lymphoma; SCNSL, secondary central nervous system lymphoma; NHL, non-Hodgkin lymphoma; IQR, interquartile range; SD, standard deviation; Ct, cycle threshold; REL, expression of miRNA relative to miR-24; NS, no significant difference.
- a Only values given for subgroups of PCNSL patients, not PCNSL versus control.
- b Patients with CRP levels >1 mg/ml excluded.
- c These data are based on a slightly larger group than the group with available CSF data.
- d Mean value, no SD reported.
microRNAs and small nuclear RNAs
Baraniskin et al (2011) were the first to study the diagnostic value of microRNA levels in CSF of PCNSL patients. They found miR-21, miR-19b and miR-92a to be diagnostic markers for CNS lymphoma and suggested that a diagnostic tree should be implemented, starting with testing for miR-21 elevation, followed by testing for miR-19 or miR-92a elevation. This diagnostic tree had a sensitivity of 95·7% and a specificity of 96·7%. An expanded study group even yielded a slightly better sensitivity, of 97·4% (Baraniskin et al, 2012a). In this second study, the value of microRNAs in serum was also investigated in a small patient group, but no significant difference in serum microRNAs between PCNSL patients and control patients was found. Another study however did show a high diagnostic value of serum miR-21 for PCNSL (area under curve [AUC] 0·930) and showed an effective distinction between PCNSL and glioblastomas (AUC 0·883) (Mao et al, 2014). A correlation between miR-21 in serum and CSF was found in this study (Pearson correlation: r2 = −0·396 P = 0·001). A more recent study combined CSF marker miR-21 with small nuclear RNA fragments of RNU2-1f in CSF (Baraniskin et al, 2016). They found a specificity of 95·7% for discriminating PCNSL patients from controls, with a sensitivity of 91·7% and an AUC of 0·987.
CXCL13
The chemokine CXCL13 has an important role in the homing of B cells (Ansel et al, 2002). Three studies analysed CXCL13 as a marker in CNS lymphoma. A relatively small study compared the value of CXCL13 in CSF of 29 patients with a combined group of PCNSL and SCNSL patients, and control groups with and without other CNS malignancies (Fischer et al, 2009). They reported no sensitivity or specificity but showed a significant difference in the CXCL13 levels in CSF of CNS lymphoma patients compared with both control groups. The largest study on diagnostic markers for CNS lymphoma included in this review (n = 220, including relevant disease controls) showed a sensitivity of 69·9% and a specificity of 92·7% for an increased CSF CXCL13 level above 90 pg/ml for CNS lymphoma (Rubenstein et al, 2013). The combination of elevated CXCL13 (>116 pg/ml) was combined elevated IL-10 (>23 pg/ml) in the diagnostic set that only applied to the patient group in which the diagnostic question is most relevant, i.e. the patients with non-human immunodeficiency virus (HIV)-associated PCNSL, the diagnostic value of these markers was even better. The sensitivity of the elevation of either CXCL13 or IL-10 above these cut-offs for the latter patient group was 84·2% and the specificity of elevation of both markers was 99·3%. A third study examined the combined diagnostic performance of CSF CXCL-13, CSF IL-10 and the apparent diffusion coefficient (ADC) on brain magnetic resonance imaging (MRI) (Mabray et al, 2016). In a cohort of 38 PCNSL and five SCNSL patients, the authors reported a sensitivity of 76·7% and a specificity of 90·9% of CSF CXCL-13 at a cut-off value of >103 pg/ml, when compared to 44 control patients with other brain tumours or tumefactive demyelinating lesions. Combination of CSF CXCL-13, CSF IL-10 and average ADC was statistically significantly superior to the single variables in this patient group, with an area under the receiver-operator characteristic (ROC) curve of 0·928.
Interleukin-6
The diagnostic value of CSF IL-6, a lymphoid growth and differentiation cytokine, at a cut-off of 4·0 pg/ml resulted in a sensitivity of 77% and a specificity of 63% (Sasayama et al, 2012). Another study on B cell PCNSL patients found a statistically significant difference of CSF IL-6, when compared to patients with non-Hodgkin lymphoma (NHL) involving the CNS and reported low sensitivity and specificity (Song et al, 2016). Ranges of IL-6 levels in patient and control groups overlapped to a large extent in both studies. Two studies analysed the diagnostic value of the IL-10/IL-6 ratio. Sasagawa et al (2015) found a sensitivity and specificity of 68·4% and 96·1% respectively with the optimal cut-off value of 2·2, while Song et al (2016) reported a much higher sensitivity of 95·6% and a specificity of 100%. The latter was the larger of the two studies (n = 102) and used a cut-off value of 0·72 (Song et al, 2016).
Interleukin-8
IL-8, a pro-inflammatory cytokine produced by several cell types, was examined by one study on B cell PCNSL, which reported low sensitivity of 31·8% and specificity of 83·1% at a cut-off value of CSF IL-8 of 117 pg/ml (Song et al, 2016).
Interleukin-10
Six different studies examined the diagnostic value of CSF IL-10, an anti-inflammatory cytokine, in the diagnosis of CNS lymphoma (Sasayama et al, 2012; Rubenstein et al, 2013; Sasagawa et al, 2015; Mabray et al, 2016; Nguyen-Them et al, 2016; Song et al, 2016). The cut-off values for CSF IL-10 that delivered the highest accuracy varied strongly among the studies, between 3·0 and 21·77 pg/ml. Two other studies on IL-10 did not report sensitivity and specificity, but did report a significant difference in the CSF IL-10 concentrations between CNSL patients and controls (Ikeguchi et al, 2017; Salmaggi et al, 2000).
Soluble interleukin-2 receptor
Interleukin-2 plays a role in T cell activation and in the maintenance of homeostatic immune responses that prevent autoimmunity (Gaffen, 2001). Its receptor is mainly expressed on activated T cells and is also partially released in the environment as sIL-2R. Four studies on sIL-2R showed diverging results on the utility of this marker in diagnosing CNS lymphoma. First, a sensitivity of 81% was found for increased sIL-2R in CSF, with a cut-off of 77 u/ml, for correct diagnosis of patients initially suspected of having a PCNSL, but specificity was only 56·7% (Sasayama et al, 2012). A second study measured sIL-2R values in serum. The difference in mean sIL-2R concentration between the PCNSL group and the control group reached significance when patients with high C-reactive protein values were excluded as a means to rule out the effect of general inflammation, but there was still a large overlap between groups, leading the authors to conclude that serum sIL-2R levels are not a sensitive and specific marker for the diagnosis of PCNSL (Kitai et al, 2013). The third study measured CSF levels and used a cut-off value of 60·4 u/ml, resulting in a much higher sensitivity of 94·7% with a specificity of 84·6% (Sasagawa et al, 2015). Highest specificity (90%) of CSF IL-2R was found by the fourth study, with a sensitivity of 83·3%, but no cut-off value was reported (Ikeguchi et al, 2017).
Soluble CD19
Muñiz et al (2014) evaluated the diagnostic value for the diagnosis of SCNSL of the concentration of sCD19 in CSF of patients with DLBCL or Burkitt lymphoma outside the CNS. CD19 is an antigen normally expressed on B-lymphocytes. They found an association between elevation of sCD19 in CSF and the presence of CNS involvement of the lymphoma in 40 patients, wherein the reference test consisted of positive CSF flow cytometry and/or highly suspicious neurological symptoms. For the cut-off value with the highest accuracy, 1·18 ng/ml, sensitivity and specificity in detecting CNS disease in this patient group were 80% and 95% respectively.
Soluble CD27
CD27 is a member of the tumour necrosis factor family and plays a role in regulating B cell activation and immunoglobulin synthesis. Kersten et al (1996) found a good diagnostic value of elevated sCD27 above 10 u/ml in CSF for meningeal involvement in acute lymphatic leukaemia (ALL) and NHL patients. In four PCNSL patients, seven out of eight CSF samples had elevated sCD27 levels (cut-off 10 u/ml), the only negative sample was obtained after dexamethasone treatment and radiotherapy. In the control group, consisting of patients with other CNS malignancies and non-lymphoid malignancies outside the CNS, all but one sample was negative. A different – methodologically flawed – study analysed the diagnostic value of detecting leptomeningeal involvement in patients with ALL, acute myeloid leukaemia and NHL (Kara et al, 2007). With a CSF cut-off value of 350 u/ml, no significant correlation was found. Murase et al (2000) investigated the utility of sCD27 levels in the diagnosis of PCNSL and demonstrated a large difference in CSF sCD27 in between 12 PCNSL patients and 30 control patients with other brain tumours. With a cut-off of 15 u/ml, all patients were classified correctly. There was great variability of cut-off levels between studies.
Tumour necrosis factor- alfa
The pro-inflammatory cytokine TNF-α plays a central role in immune responses and it is suggested that serum levels are correlated with prognosis in DLBCL patients (Lech-Maranda et al, 2010). For this reason, Song et al (2016) examined the diagnostic value of CSF TNF-α in B cell PCNSL patients, but found low sensitivity and specificity of 59·1% and 57·1% respectively, with a cut-off value of 5·3 pg/ml.
Beta-2-microglobulin
Several studies have focused on the potential of B2M, a component of major histocompatibility complex class I molecules, as a marker of CNS involvement in lymphoid malignancies. Only studies that reported separate data for primary or secondary CNS lymphoma are presented in Table 1. The best accuracy for the diagnosis PCNSL was obtained with a CSF B2M cut-off value of 2·06 mg/l, with an AUC of 0·934 (Sasayama et al, 2012). A slightly higher cut-off value of 2·4 mg/l in CSF was used to differentiate CNS lymphoma from non-lymphomas with a sensitivity and specificity of 89·4% and 88·5% respectively (Sasagawa et al, 2015). A CSF cut-off value of 2·56 mg/l was found to have the highest accuracy in detecting CNS involvement in patients with DLBCL and Burkitt lymphoma (Muñiz et al, 2014). Further, a small study showed a significant difference in B2M CSF levels between NHL patients with and without CNS involvement (Mavlight et al, 1980). In a slightly larger study, this difference in CSF B2M levels between NHL patients with and without CNS involvement was also present, but there was a large overlap between the two groups (3·9 ± 3·5 mg/l and 1·7 ± 1·0 mg/l for patients with and without CNS involvement of NHL, respectively). Specificity and sensitivity derived from these data were only 71% and 67% respectively for a cut-off of 1·9 mg/l (Ernerudh et al, 1987). Finally, the diagnostic value for B2M was also examined in a group of NHL and ALL patients with suspected CNS involvement (Kersten et al, 1996). The authors found that the CSF/serum ratio provided a slightly better discrimination between the presence and absence of CNS involvement than CSF levels of B2M alone. However, they also reported that the B2M ratio was also increased in 9 of 40 samples from 32 patients with other CNS malignancy or non-lymphoid malignancy without CNS involvement.
Anti-thrombin III
Roy et al (2008) identified 80 proteins in the CSF of 24 patients with CNS lymphoma compared to 77 control patients without CNS malignancy and evaluated the diagnostic value of one of these markers, antithrombin III (ATIII). ATIII is an enzyme that inactivates thrombin (coagulation factor IIa). Elevation of ATIII above 1·2 μg/ml was 75% sensitive and 98·7% specific in this study population. When normalized for total CSF protein, the ATIII concentrations in patients with CNS lymphoma were still significantly higher than in control patients (P < 0·015). However, the mean CSF value of ATIII in of 13 patients with CNS metastasis of breast and lung cancer was also elevated (2·6 μg/ml vs. 1·68 μg/ml in CNS lymphoma patients).
A statistically significant difference in median CSF ATIII concentration was also found in another group of lymphoma patients compared to non-neoplastic neurological disorders (P = 0·002), and PCNSL patients on the one hand compared to SCNSL or patients without CNS lymphoma lesions on the other (P = 0·006) (Kuusisto et al, 2015). However, the distribution of ATIII concentrations overlap between the different groups, indicating a poor discriminative value of ATIII.
sTACI and sBCMA
Transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and B cell maturation antigen (BCMA) are receptors involved in the B cell homeostasis system. It was recently demonstrated that both receptors also exist in a soluble form (Thaler et al, 2017). A recent study on PCNSL patients described superior diagnostic value of sTACI over sBCMA in CSF (Thaler et al, 2017). The highest specificity (96·7%) was reported for the combination of sTACI and sBCMA, with cut-off values of 68·4 and 460 pg/ml respectively, and with a sensitivity of 63·9%.
Neopterin
Neopterin is a non-specific marker of the type 1 T-helper cell derived immune response. It is produced by human monocytes/macrophages upon stimulation with interferon-γ. Elevated neopterin levels in serum, CSF and urine have been associated with different infectious and inflammatory diseases, such as HIV infections, but also with auto-immune diseases and malignant tumours (Murr et al, 2002). Viaccoz et al (2015) compared CSF neopterin levels in PCNSL patients with patients with other brain tumours or inflammatory CNS lesions. They found a sensitivity of 96% and a specificity of 92% at a cut-off value of 10 nmol/l.
Osteopontin
Another pro-inflammatory cytokine, osteopontin, is associated with progression, metastatic spread and poor prognosis in many malignancies (Strehlow et al, 2016). In a combined patient group of PCNSL and SCNSL, CSF osteopontin showed a sensitivity of 87% and a specificity of 86%, compared to control patients, with a cut-off value of 400 ng/ml (Strehlow et al, 2016). Serum osteopontin demonstrated poor diagnostic value in this population.
Quality of the studies
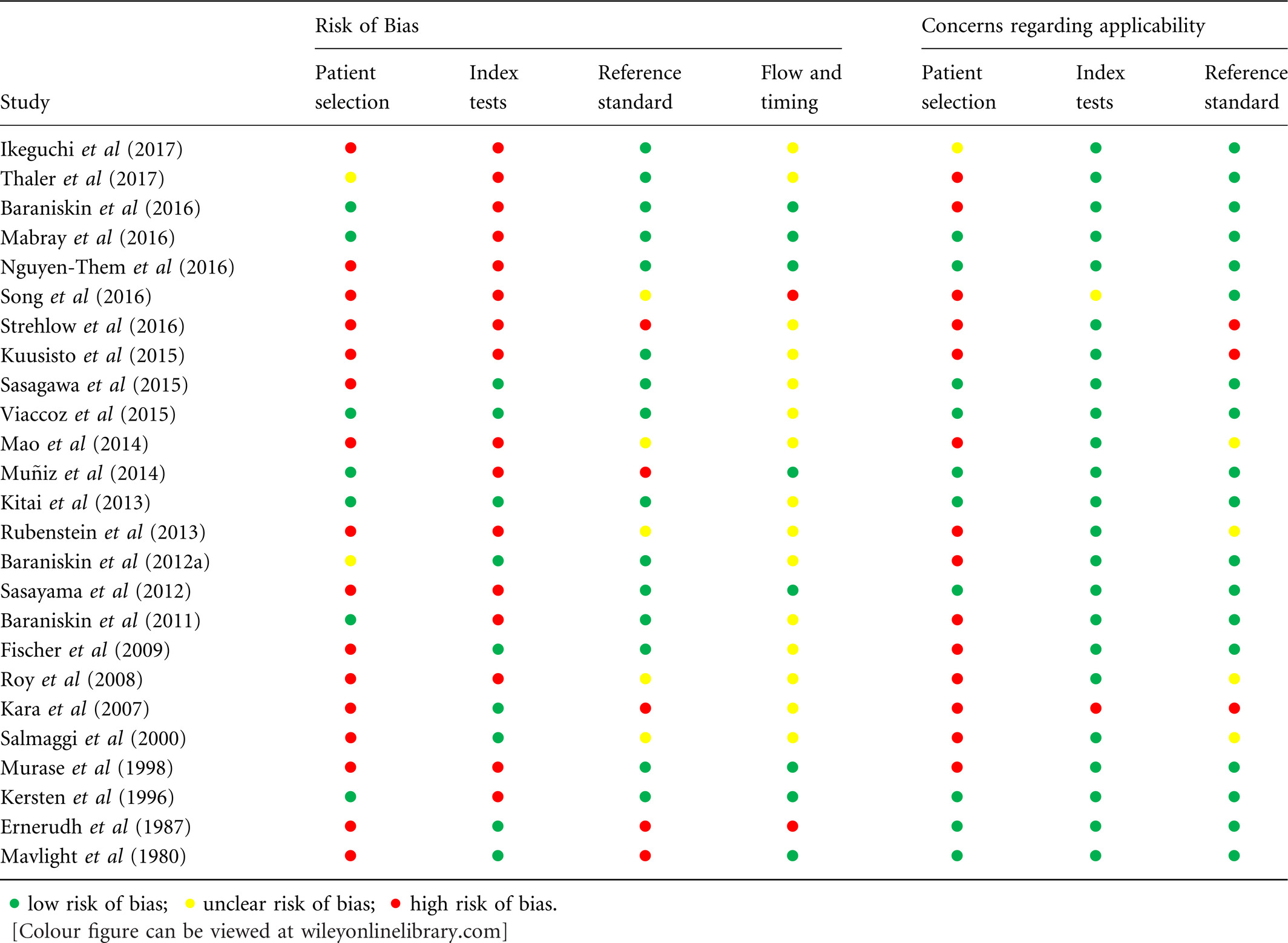
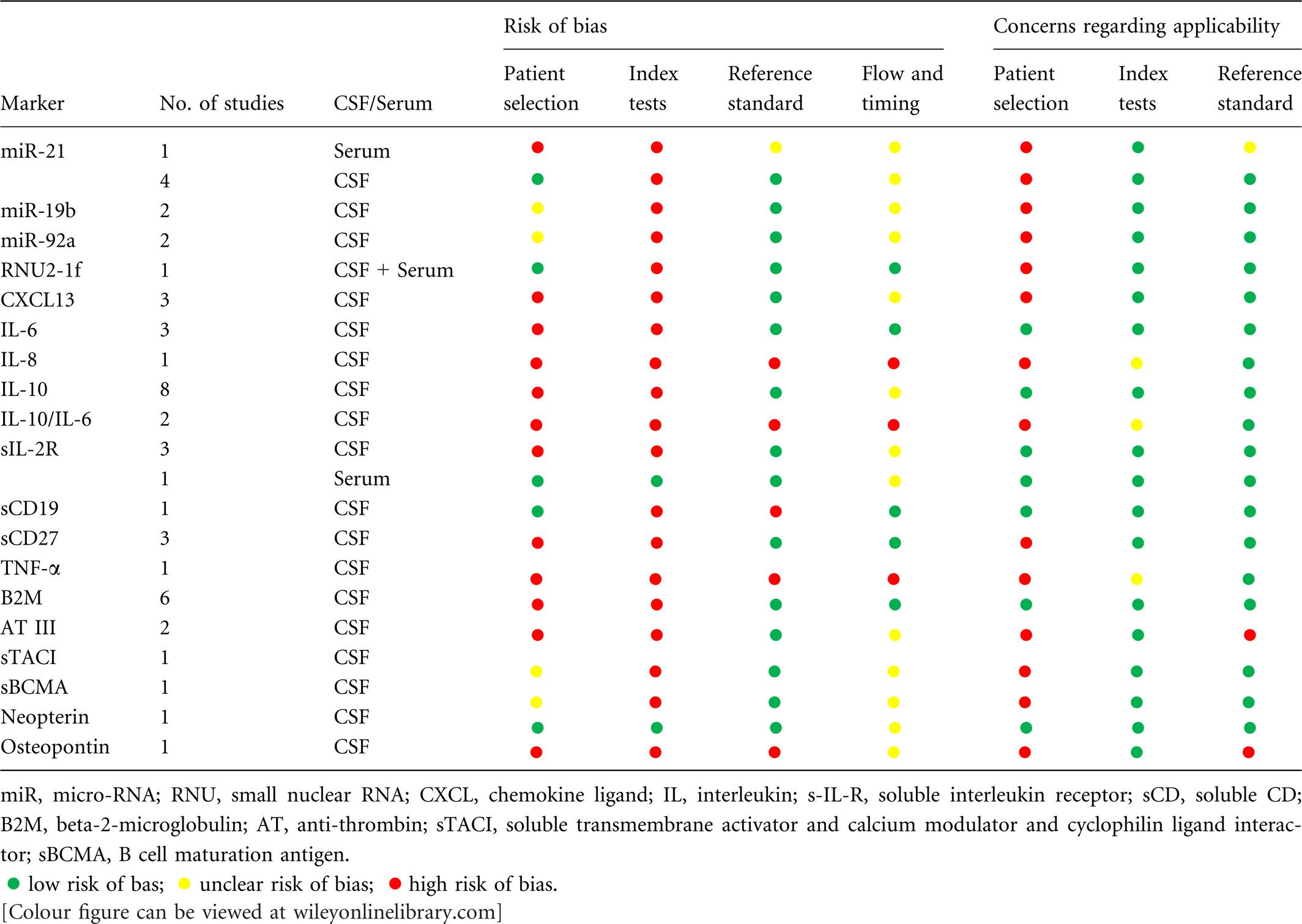
The quality of the studies included in this systematic review is presented in Tables 4 (per study) and 5 (per marker). All studies were at risk of bias, due to the risk of (i) bias in patient selection and (ii) bias introduced by post-hoc threshold determination in the index test. Only nine studies reported on a consecutive sample of patients (Kersten et al, 1996; Baraniskin et al, 2011, 2016; Kitai et al, 2013; Muñiz et al, 2014; Mabray et al, 2016; Nguyen-Them et al, 2016; Ikeguchi et al, 2017; Thaler et al, 2017), therefore the remaining studies were at high or unclear risk of bias on this item. The study by Baraniskin et al (2012a) did not report how additional patients [above those already included in Baraniskin et al (2011)] were included, so the risk of bias in this study was scored as unclear. Kara et al (2007) reported a patient sample of seven NHL patients, of whom only one had leptomeningeal involvement. The majority of patients was diagnosed with leukaemia, which did not correspond to the research question. Concerning the index test, only five studies (Ernerudh et al, 1987; Baraniskin et al, 2012a; Kara et al, 2007; Sasagawa et al, 2015; Viaccoz et al, 2015) used pre-specified cut-off values in determining diagnostic value and seven studies (Mavligit et al, 1980; Salmaggi et al, 2000; Fischer et al, 2009; Kitai et al, 2013; Kuusisto et al, 2015; Baraniskin et al, 2016; Ikeguchi et al, 2017) did not use a threshold, but compared mean levels of the marker in patient and control group. Hence, risk of bias concerning the index test was high in the other thirteen studies. There was no reference standard described in six studies (Murase et al, 2000; Kara et al, 2007; Roy et al, 2008; Rubenstein et al, 2013; Mao et al, 2014; Strehlow et al, 2016) and there was possible risk of bias in the way the used reference standard classified the presence or absence of CNS lymphoma in three studies (Ernerudh et al, 1987; Mavligit et al, 1980; Muñiz et al, 2014).
There were concerns regarding the applicability of data from 15 of the reviewed articles (Murase et al, 2000; Salmaggi et al, 2000; Kara et al, 2007; Roy et al, 2008; Fischer et al, 2009; Baraniskin et al, 2011, 2012a, 2016; Rubenstein et al, 2013; Mao et al, 2014; Kuusisto et al, 2015; Song et al, 2016; Strehlow et al, 2016; Ikeguchi et al, 2017; Thaler et al, 2017). These concerns resulted from the composition of the control groups, which either contained irrelevant diagnoses or lacked important differential diagnoses (‘lymphoma mimics’). Control groups either consisted of healthy subjects (Mao et al, 2014; Strehlow et al, 2016), patients with non-neurological disease (Kuusisto et al, 2015) or neurological disease that are usually not included in the differential diagnosis of PCNSL (e.g. tension-type headache, Alzheimer) (Baraniskin et al, 2011, 2012a, 2016), or lacked patients with CNS malignancies (Roy et al, 2008; Fischer et al, 2009) or CNS inflammation (Murase et al, 2000; Salmaggi et al, 2000). Rubenstein et al (2013) analysed patients with SCNSL and PCNSL as one group. However, in clinical practice the diagnostic question as well as the differential diagnosis for these conditions is different, resulting in concerns regarding the applicability of the patient selection in this study as well as five other studies (Roy et al, 2008; Fischer et al, 2009; Mabray et al, 2016; Strehlow et al, 2016; Ikeguchi et al, 2017).
Discussion
In this systematic review, we searched for markers in CSF or serum with potential value for the diagnosis of CNS lymphoma. We found evidence for the diagnostic utility of the following markers: miR-21, miR-19b, miR-92a, RNU2-1f, CXCL-13, IL-6, IL-8, IL-10, sIL-2R, sCD19, sCD27, TNF-α, B2M, ATIII, sTACI, sBCMA, neopterin and osteopontin in CSF, and for miR-21, sCD27, and B2M in blood.
Optimal clinical value
In the interpretation of studies of diagnostic test accuracy, the specific clinical situation dictates what the optimal diagnostic yield would be. For a certain diagnosis of CNS lymphoma, specifically PCNSL, high specificity is more important than sensitivity, to avoid false-positive diagnoses. Conversely, as a screening tool that should detect all possible cases of (P)CNSL, high sensitivity is most important. In current practice, clinical and radiological features already raise suspicion on CNS lymphoma. A test that could replace the need for biopsy to confirm the diagnosis of CNS lymphoma should ideally have no false positive results, so that the diagnosis of CNS lymphoma is certain and treatment can be initiated. High specificity is therefore the important test quality. A specificity of ≥90% – representing the potential to be developed into a replacement for current reference tests – was reported for combined sTACI and sBCMA (Thaler et al, 2017), RNU2-1f (Baraniskin et al, 2016), miR-21 (Baraniskin et al, 2016), the diagnostic tree of miR-21, miR-19b and miR-92a (Baraniskin et al, 2011), neopterin (Viaccoz et al, 2015), sCD19 (Muñiz et al, 2014), sCD27 (Kersten et al, 1996; Murase et al, 2000), CXCL-13 (Rubenstein et al, 2013; Mabray et al, 2016), IL-10 (Sasayama et al, 2012; Rubenstein et al, 2013; Sasagawa et al, 2015; Mabray et al, 2016; Song et al, 2016), IL-10/IL6 ratio (Sasagawa et al, 2015; Song et al, 2016), B2M (Sasayama et al, 2012; Muñiz et al, 2014), and AT-III (Roy et al, 2008). Muñiz et al (2014) however only studied patients with SCNSL and in several of the available studies (Murase et al, 2000; Roy et al, 2008; Rubenstein et al, 2013; Baraniskin et al, 2016; Song et al, 2016; Thaler et al, 2017), concerns regarding applicability data were high because of patient selection issues.
Considerations on individual markers
MicroRNAs (miR) and small nuclear RNAs (snRNA) play key roles in many (haematological) cellular pathways and dysfunction of microRNAs is found to be a feature of malignancies (Calin & Croce, 2006; Lawrie, 2007). Mao et al (2014) reported a large area under the ROC-curve for levels of miR-21 in serum; Baraniskin et al (2012a), however, reported no elevation of miR-21 in serum compared to controls. This might be due to the different composition of the control groups. In Mao et al (2014) the control group contained healthy people, which does not reflect clinical practice. Since MiR-21 was also found to be elevated in the serum of patients with glioblastoma (Baraniskin et al, 2012b), future studies on miR-21 in CSF should compare PCNSL patients to adequate disease controls including glioblastoma patients. Mao et al (2014) did report diagnostic value for miR-21 in serum when CNS lymphoma patients were compared to patients with glioblastoma.
Rubenstein et al (2013) found good diagnostic accuracy for CXCL13 in CSF in a large-scale study. Importantly, this marker was also elevated in half of the patients with brain metastases of breast cancer. Clinical use of this marker may lead to incorrect diagnosis of PCNSL in patients with CNS metastases of breast cancer, specifically when there is no known diagnosis of breast cancer. Mabray et al (2016) endorses the good diagnostic value of CXCL13 in CSF, especially when it is combined with CSF IL-10 and average ADC on brain MRI. Of note, CXCL13 is often positive in cases of neuroborreliosis, and has in fact been identified as a useful marker to discriminate neuroborreliosis from other neuro-inflammatory diseases (Schmidt et al, 2011). Neuroborreliosis, however, is usually not one of the main differential diagnoses for CNS lymphoma.
Individual interleukins were examined in various studies, but none of them reached good diagnostic value, due to wide variation in cut-off value, low sensitivity or low methodological quality of the investigating study. Blood or CSF interleukin levels can also be elevated in other conditions included in the differential diagnosis of CNS lymphoma. CSF IL-10 for example, is found to be elevated in neuro-inflammatory and –infectious diseases (Wang et al, 2017). IL-6 secretion is stimulated in Waldenström macroglobulinaemia and increased levels can be detected in the blood of patients with Bing Neel syndrome (Elsawa et al, 2011). Nevertheless, the ratio of IL-10 and IL-6 is already used in differentiating intraocular lymphoma (ratio > 1) from uveitis (Kimura et al, 2012). Sasagawa et al (2015) found high specificity, but relatively low sensitivity, of this ratio in CSF for distinguishing CNS lymphoma from other CNS brain lesions; patients with inflammatory diseases were scarce in the control group. In line with the findings in ocular disease, the IL-10/IL-6 ratio would also be expected to be lower in inflammatory CNS diseases than in CNS lymphoma. However, Sasayama et al (2012) found moderate diagnostic value for elevated IL-6 levels ≥4·0 pg/ml for CNS lymphoma (regardless of IL-10 level); this seemingly counterintuitive finding may be due to the fact that the control group consisted mainly of patients with other CNS malignancies and only contained two patients with CNS inflammation. Song et al (2016) did include a significant number of patients with inflammatory CNS diseases in their control group and found high specificity as well as high sensitivity of the IL-10/IL-6 ratio, however the cut-off value (a ratio of 0·72) was not pre-determined and differed from previous reports.
Although sCD27 showed good diagnostic value in three studies that compared PCNSL with other CNS disease, these studies suffered from inadequate control groups and small numbers of PCNSL patients (two studies combined PCNSL with other haematological malignancies) (Kersten et al, 1996; Murase et al, 2000; Kara et al, 2007).
sCD19 has only been evaluated as a marker of CNS involvement in systemic DLBCL or BL, compared with patients who had DLBCL or BL without CNS involvement as a control group. The diagnostic value of this marker still needs to be determined for patients with primary CNS lymphoma, with other brain tumours and CNS inflammation as a control group.
B2M has already been identified as a biomarker for a broader spectrum of lymphoid malignancies (Pudek et al, 1985; Ernerudh et al, 1987; Oberg et al, 1987; Mavligit et al, 1980). This may explain the difference in diagnostic values found among the studies. Two studies reported good diagnostic value of B2M in CSF when PCNSL patients were compared to patients with other brain malignancies (Sasayama et al, 2012; Sasagawa et al, 2015). The comparison of SCNSL patients with other lymphoid malignancies without CNS involvement, however, resulted in poor diagnostic values (Ernerudh et al, 1987; Kersten et al, 1996; Muñiz et al, 2014). None of the studies on B2M included a significant number of patients with neuro-inflammation in their control group.
Great differences in concentration of the sIL-2R between PCNSL patients and controls was reported by Sasayama et al (2012) when measured in CSF. Moreover, Sasagawa et al (2015) and Ikeguchi et al (2017) endorse this diagnostic value of CSF sIL-2R, although their patient groups were small and also comprised SCNSL. Kitai et al (2013) however showed that sIL-2R was not a good diagnostic marker when measured in serum, due to the elevation of sIL-2R in patients with inflammatory disease. Serum sIL-2R is also a marker of disease activity in patients with sarcoidosis and a criterium in haemophagocytic lymphohistiocytosis, and in CSF it is frequently used as an indicator for neurosarcoidosis (Grutters et al, 2003; Petereit et al, 2010). There were only a few patients with inflammatory disease in the control groups of Sasayama et al (2012), Sasagawa et al (2015) and Kitai et al (2013). The majority of control patients from Ikeguchi et al (2017) had multiple sclerosis, however, the authors did not make it clear which control group was used for calculating the diagnostic value. It should be further investigated whether sIL2R concentration in CSF can help to distinguish between CNS lymphoma and inflammatory disease.
Antithrombin III could not discriminate between CNS lymphoma and cerebral metastasis (Roy et al, 2008) or non-neoplastic neurological diseases (Kuusisto et al, 2015) and is likely to be a non-specific marker of blood-brain barrier disruption (Zetterberg et al, 2009). However, it might be useful in the demonstration of CNS involvement in peripheral NHL (i.e. SCNSL), although this has not yet been validated.
The diagnostic value of sTACI and sBCMA was examined by one study of moderate methodological quality (Thaler et al, 2017). sTACI showed superior diagnostic value over sBCMA and combination of the two biomarkers in CSF revealed highest specificity, at the expense of a lower sensitivity.
Viaccoz et al (2015) were the first to describe neopterin as a biomarker for PCNSL, as it previously had been used as a marker for several infectious and inflammatory diseases. This low-bias study shows promising diagnostic accuracy of neopterin for the differentiation between PCNSL and other brain tumours, pseudotumoural inflammatory lesions or nontumefactive inflammatory CNS disorders. However, sample sizes were limited and, given that high CSF neopterin levels are reported in demyelinating disease (Viaccoz et al, 2015), larger studies are needed, including patients with pseudotumoural infectious and inflammatory disease.
Osteopontin showed good potential as a CSF biomarker for CNSL, but methodological quality of the study was very poor and therefore these results have to be interpreted with caution (Strehlow et al, 2016).
Circulating cell-free DNA in blood has been identified as a biomarker for tumour burden and malignant progression in cancer patients (Schwarzenbach et al, 2011). Recently, this biomarker was also used to identify somatic mutations of PCNSL in blood or CSF, which has potential for individualizing antineoplastic treatments (Fontanilles et al, 2017), and possibly as a diagnostic tool; however, no diagnostic studies have been published thus far.
In addition to the diagnostic value of the above-mentioned biomarkers, some authors also emphasized the possible prognostic value of these markers in CNS lymphoma. Elevated microRNA's were negatively related to prognosis in several studies (Baraniskin et al, 2011, 2012a; Mao et al, 2014) as well as RNU-21f (Baraniskin et al, 2016), TNF-α (Lech-Maranda et al, 2010; Dlouhy et al, 2017) and sCD19 (Muñiz et al, 2014). Evidence for CSF IL-10 was conflicting (Salmaggi et al, 2000; Lech-Maranda et al, 2010; Mizowaki et al, 2015; Nguyen-Them et al, 2016; Sasayama et al, 2016; Wang et al, 2017). The association between IL-6 and sIL-2R levels and prognosis of CNS lymphoma patients is not unambiguously reported (Sasayama et al, 2016; Dlouhy et al, 2017). More evidence of sufficient quality is needed to evaluate the prognostic value of these markers.
Limitations
This review is based on mostly retrospective studies; only two studies were prospective and one study was partially prospective. The number of studies about each individual marker were low and most markers were studied in small patient cohorts. We could not perform a formal meta-analysis of diagnostic test accuracy for each marker due to the differences between studies in sample selection (specifically the selection of control groups), in thresholds for each marker and in reporting of outcome measures. Quality assessment of the included studies by means of QUADAS-2 demonstrated that all studies were at risk of bias and there was concern regarding applicability of the selected patients and controls in 15 studies (Murase et al, 2000; Salmaggi et al, 2000; Kara et al, 2007; Roy et al, 2008; Fischer et al, 2009; Baraniskin et al, 2011, 2012a, 2016; Rubenstein et al, 2013; Mao et al, 2014; Kuusisto et al, 2015; Song et al, 2016; Strehlow et al, 2016; Ikeguchi et al, 2017; Thaler et al, 2017). Due to these findings, it cannot be determined yet whether the reported diagnostic values of the markers will also hold true in clinical practice. Although some studies reported on the combination of different biomarkers (Baraniskin et al, 2011, 2012a; Rubenstein et al, 2013; Sasagawa et al, 2015; Mabray et al, 2016; Song et al, 2016; Thaler et al, 2017), data on the combined use of several of these markers is limited. It is suggested that biomarker profiles of patients, based on several different biomarkers, could show better diagnostic accuracy than single markers (Thijs et al, 2017).
Conclusion
Available evidence on diagnostic markers for the diagnosis of CNS lymphoma in blood or CSF is of limited quality. Methodological issues originate from mostly retrospective small-scale studies with heterogeneous sample selection and test definitions, leading to high risk of bias for all markers but one.
miR-21, miR-19b, miR-92a, RNU2-1f, CXCL-13, IL-10, sIL-2R, sCD19, sCD27, B2M, cTACI and sBCMA, neopterin and osteopontin in CSF and miR-21, and the sIL-2R in blood are possible diagnostic markers for both primary and secondary CNS lymphoma. ATIII has limited utility due to the poor ability to discriminate PCNSL from other malignancies. CXCL-13, B2M and neopterin in CSF show the highest potential to form a diagnostic marker for CNS lymphoma. However, we do not yet recommend application of these markers in clinical practice because of the current insufficient level of empirical evidence. As of now, brain biopsy still remains the gold standard.
We do, however, emphasize the potential of this relatively non-invasive diagnostic tool. The diagnostic value of these markers should be further studied in prospective cohort studies with sufficient numbers of consecutively collected patients, adequate control groups and predefined test thresholds, before they can be used in clinical practice. Moreover, validation of these markers in consecutive cohorts, with simultaneous measurement of several or all of the identified candidate-markers is recommended. When diagnostic value of these markers, or clusters of several markers, is confirmed, stereotactic biopsy might not be longer necessary in some patients or may be hastened in other patients. These changes could lead to faster diagnosis, fewer cases of biopsy-related morbidity and, possibly, better survival and quality of life.
Authors contributions
All authors contributed to analysis and writing of the manuscript and approved its final version. LCAS and TJS designed the study. LCAS and AW acquired data. LCAS, AW and TJS first analysed the data.
Funding
No funding was received for this research.
Competing interests
The authors have no competing interests.