Unexplained thrombocytosis: association of Baltimore polymorphism with germline MPL nonsense mutation
A 44-year-old female patient born in northern Africa presented with a thrombocytosis (platelet count 610 × 109/l) with no causes of reactive elevation of platelet counts, and no mutation in JAK2 or CALR genes or BCR-ABL1 transcript. Red blood cell and leucocyte counts were normal. As MPL W515L/K mutations are present in 5–10% of JAK2 V617F and CALR exon 9 negative Essential Thrombocythaemia (ET) patients (Klampfl et al, 2013), a mutation of exon 10 of the MPL gene was sought using the MPL Mutascreen® kit (Qiagen™, Hilden, Germany). We were surprised to observe an abnormal feature of the normalized fluorescence on the mutant axis (Fig 1A). In our experience, such a fluorescence profile may be linked to the presence of rare mutants such as MPL W515A or W515S (Chaligné et al, 2008). The entire coding regions of the MPL gene were thus sequenced and we identified two different MPL heterozygous variants (Fig 1B): a stop codon at position 515 (p.W515*) and a K39N variant. The K39N variant, also known as the Baltimore polymorphism, is associated with thrombocytosis when present in a homozygous state (Moliterno et al, 2004). However, Sanger sequencing on DNA extracted from the patient's nails revealed the heterozygous status and germline origin of both the K39N variant and the MPL mutation.
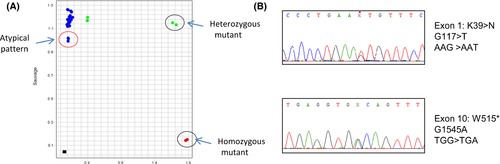
The presence of truncating mutations similar to the p.W515* mutation in the MPL gene have not been associated with thrombocytosis to date, but with congenital amegacaryocytic thrombocytopenia (CAMT), a rare disease characterized by severe thrombocytopenia developing during infancy with later evolution to pancytopenia (Ihara et al, 1999). CAMT is an autosomal recessive disease in which MPL mutations are considered as the causative origin of the disease (Geddis, 2011). Mutations have been found at different locations along the MPL gene sequence in this disorder, including missense, nonsense or splice site mutations. The vast majority of CAMT cases are due to homozygous or compound heterozygous mutations in MPL (Ballmaier & Germeshausen, 2011). To better understand the distribution of these mutations on the different alleles and determine how an MPL-CAMT-associated mutation could explain the thrombocytosis observed in this patient, we analysed the cells of the patient's daughter. Despite normal blood cells counts, the heterozygous MPL p.W515* truncating mutation was present in DNA from the patient's daughter. The K39N variant was not detected. These results demonstrate that both variants were on different alleles and thus acting in trans, but also that the daughter received the MPL gene truncating mutant from her mother and a wild type allele from her father). This analysis confirms that this heterozygous truncating mutation of the thrombopoietin receptor (THPO-R, also termed MPL) has no or only a mild impact on the platelet count and is not sufficient per se to give rise to either thrombocytosis or CAMT.
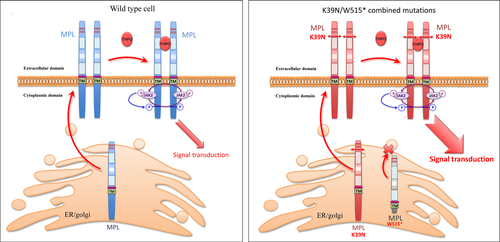
CAMT-associated truncating mutations in MPL principally lead to defective THPO-R presentation on the cell surface, leading to unresponsiveness to thrombopoietin (THPO) (Ballmaier et al, 2001, 2015; Varghese et al, 2014). However, no report in the literature or in the COSMIC (Catalogue of somatic mutations in cancer) database refers to the p.W515* mutation, which encodes a receptor lacking the intracellular domain. It may be surmised that in this patient, similar to CAMT patients, the truncated receptor may not be addressed at the membrane. Thus, for this patient, the only MPL proteins addressed at the cell-surface are the MPL proteins encoded by the K39N variant gene and may thus mimic the homozygous K39N MPL receptor at the protein level, explaining the thrombocytosis observed in this patient (Fig 2).
In this context, genetic advice is not easy to deliver to the patient's daughter for her future children. Indeed according to the father's MPL genotype, several consequences may occur in offspring that receive the MPL W515* truncated allele from the mother. Although the most likely consequence would be that a potential father may harbour 2 normal alleles of the MPL gene and thus offspring would have normal platelet counts, it cannot be ruled out that the children may suffer from a very severe CAMT phenotype if the father transmits a CAMT-associated mutation, or from thrombocytosis should the father transmit a K39N variant.
In conclusion, we report the presence of two germline mutations, one ‘pro-thrombocytopenic’ and one ‘pro-thrombocythaemic’, on two different alleles of the MPL gene in a patient with thrombocytosis. This situation may exist with other cytokine or growth factor receptors. This study emphasizes the importance of direct sequencing of all the coding regions of the MPL gene for patients with unexplained thrombocytosis.
Author contributions
EV, GL and CC performed molecular analyses. FT contributed clinical data. BC, CC and SG designed the study and wrote the paper. All authors approved the final version of the manuscript.




