Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, donor selection algorithms and conditioning protocols
Summary
Allogeneic haemopoietic stem cell transplantation offers a potentially curative treatment option for a wide range of life-threatening malignant and non-malignant disorders of the bone marrow and immune system in patients of all ages. With rapidly emerging advances in the use of alternative donors, such as mismatched unrelated, cord blood and haploidentical donors, it is now possible to find a potential donor for almost all patients in whom an allograft is indicated. Therefore, for any specific patient, the transplant physician may be faced with a myriad of potential choices, including decisions concerning which donor to prioritize where there is more than one, the optimal selection of specific umbilical cord blood units and which conditioning and graft-versus-host disease prophylactic schedule to use. Donor choice may be further complicated by other important factors, such as urgency of transplant, the presence of alloantibodies, the disease status (homozygosity or heterozygosity) of sibling donors affected by inherited disorders and the cytomegalovirus serostatus of patient and donor. We report UK consensus guidelines on the selection of umbilical cord blood units, the hierarchy of donor selection and the preferred conditioning regimens for umbilical cord blood transplantation, with a summary of rationale supporting these recommendations.
Umbilical cord blood (UCB) is an established alternative source of haematopoietic stem cells (HSC) for allogeneic transplantation when suitable human leucocyte antigen (HLA)-matched sibling or well matched unrelated donors are unavailable. Over 35 000 UCB transplants have now been performed worldwide and there are over 630 000 cord blood units (CBU) currently stored in international cord blood blanks. The emerging experience of umbilical cord blood transplantation (UCBT) and, more recently, haploidentical donor transplants have extended access to HSC transplantation (HSCT) in almost all those previously precluded. Consequently, the optimal selection of a donor or specific CBU for an individual patient has become increasingly complex.
Here we report new UK consensus recommendations for the method of selection of individual UCB units, the role of cord blood transplantation (CBT) within an overall donor selection strategy and the choice of conditioning regimens for CBT. These recommendations are primarily based on retrospective comparative studies and expert opinion given the lack of randomized trials comparing different donor choices.
Cord blood transplantation in the UK
The inclusion of UCBT into routine practice in the UK was initially slow, despite the early establishment of the London Cord Blood Bank in 1996. The reasons for this included lack of a national strategy, lack of clinical trials, concern regarding the safety of UCBT in adults prior to 2004 and well established, successful alternative donor strategies, such as the use of T-cell depletion in adults (permitting selection of mismatched donors) and haploidentical regimens in children.
To address these issues the British Society of Blood and Marrow Transplantation (BSBMT) Cord Blood Working Group (CBWG) was established in 2008. Following a successful workshop, first recommendations for incorporating CBT into standard UK transplantation practice were published in January 2009 (Shaw et al, 2009). Two National Cancer Research Institute (NCRI) adopted clinical trials investigating the efficacy and safety of reduced intensity conditioning (RIC) and myeloablative conditioning (MAC) UCBT were opened in 2009 and 2010 respectively, funded by the Sue Harris Trust.
Up until the end of 2006, only 183 UCBTs had been performed in the UK. Over the following 5 years, there was a rapid expansion in the number of procedures, peaking at around 90 per year and remaining stable thereafter (http://bsbmt.org/2005-activity/). Although UCBT still comprises only around 6% of all UK allograft activity, it is recognized that a substantial number of patients might benefit if UCBT was used more widely (NHS Blood and Transplant, 2014). The UK is an increasingly ethnically diverse society (http://www.migrationinformation.org/datahub/countrydata/data.cfm). Lown et al (2013) recently demonstrated that a fully matched unrelated donor can be identified for approximately 70% of patients of white Northern European (WNE) descent but only 20% of non-WNE descent in the UK. The availability of mismatched unrelated, cord blood and haploidentical donors have increased access to transplant to those of non-WNE descent, such that the rates of transplantation in both groups is now comparable. However, the time from initiation of donor search to transplant is still longer in those of non-WNE heritage, primarily due to a protracted confirmatory typing stage.
In the light of these on-going challenges and emerging data regarding optimum graft selection and clinical outcomes, a second CBWG symposium was held at the Royal College of Pathologists in October 2013, funded by Anthony Nolan and the National Health Service Blood and Transplant. The meeting had representation from senior transplant physicians, UK cord blood banks and scientists from across the UK. The aim was to produce a second consensus statement with a focus on UCB unit selection, donor selection strategy and conditioning regimens. Subsequent to this meeting, the provisional recommendations have been discussed widely within the paediatric and adult transplant communities, the UK cord blood banking communities and the British Society for Histocompatibility and Immunogenetics. The final consensus recommendations are presented below.
Selection of umbilical cord blood units
HLA-matching and cell dose
Conventionally, HLA-matching of CBUs for HSCT has used low/intermediate resolution typing for HLA-A and -B (antigen) and high resolution typing for HLA-DRB1 (allele). Using these criteria, the critical association between CBU cell dose and degree of HLA-matching with successful engraftment following CB transplantation is well established (Gluckman et al, 1997; Rubinstein et al, 1998).
Increasing HLA disparity is associated with inferior neutrophil and platelet engraftment and an increased risk of acute graft-versus-host disease (GVHD) and transplant-related mortality (TRM) (Barker et al, 2010). Increasing cell dose is associated with more favourable outcomes and may ameliorate, although not completely overcome, the deleterious impact of each HLA mismatch (Barker et al, 2010).
In bone marrow (BM) and peripheral blood HSCT, the importance of allelic disparity of HLA-class I (A, B and C) and class II (mainly DRB1) loci in determining transplant outcomes is well recognized. More recently, a joint Center for International Blood and Marrow Transplant Research and Eurocord study analysed the effect of high resolution typing (HLA-A, -B, -C, and -DRB1) on the outcomes of 1658 myeloablative single UCBTs for haematological malignancy, primarily in children (Eapen et al, 2014). Day 28 neutrophil recovery was significantly lower for transplants mismatched at three or more alleles compared to UCBT with fully matched CBU (8/8) or UCB grafts mismatched at one/two alleles. NRM was associated with HLA-matching, with the lowest risk observed in those transplants fully matched at HLA-A, -B, -C, and -DRB1 (8/8). Single allele mismatches at HLA-A, -C or -DRB1 were associated with increased NRM {Hazard ratio (HR) 3·05 [95% confidence interval (CI), 1·52–6·14], P = 0·002; HR 3·04 (95% CI, 1·28–7·20), P = 0·01; HR 2·93 (95% CI, 1·38–6·25), P = 0·005 respectively}. Of particular note, using CBUs with total nucleated cell (TNC) dose <3·0 × 107/kg had higher NRM, independent of HLA-matching. However, further increases in TNC above the minimum threshold of 3·0 × 107/kg were not associated with a further reduction in NRM. In all groups of HLA match (4/8, 5/8, 6/8, 7/8) a collected TCD dose <3·0 × 107/kg was associated with 1-year non-relapse mortality of around 40%. However in the group of 4/8 or 5/8, patients transplanted with a cell dose higher than 5·0 × 107/kg, the 1-year NRM was 20% which was comparable to patients receiving a CB graft of HLA 6/8 or 7/8 with a cell dose of greater than 3·0 × 107/kg. Patients given a CB graft of 3/8 had a high NRM independent of the cell dose.
For non-malignant conditions, the interaction between cell dose and HLA-matching is less clear, perhaps reflecting the biology in these disorders, the effects of previous treatment and the relative paucity of outcome data compared to haematological malignancy (Rocha & Gluckman, 2009). Patients with chemotherapy-naïve conditions, e.g. haemoglobinopathies, generally have a more cellular marrow and are less immunosuppressed prior to transplantation, while heavily pre-transfused recipients are more likely to have anti-HLA-antibodies. However, cell dose and HLA match are critical predictors of outcome, with a higher cell dose required than in the malignant setting (Rocha & Gluckman, 2009).
Additional markers of cell content
The optimal timing and measurement of CB cell content still remains to be fully established. Most published studies have analysed the impact of TNC dose prior to freezing or after thawing. However, when selecting a unit based upon cell counts prior to freezing, it should be noted that there is around a 20% nucleated cell loss after thawing (Laroche et al, 2005). CD34+ cell count may be a better marker of cell content because it more accurately reflects the HSC and haematopoietic progenitor cells (HPC). However, historical differences in CD34 quantification methodologies and availability make interbank comparison difficult, especially with older units. Therefore, when CD34+ cell counts are available these should be taken into consideration, but cannot yet replace TNC as the primary cell dose criterion.
Another important consideration is the association of ‘viable’ TNC or CD34+ cells infused with clinical outcomes (Barker et al, 2009; Purtill et al, 2014). However, as current methodologies to measure cell viability vary significantly between different transplant centres, multi-centre retrospective analyses of CB cell viability remain difficult. Granulocyte-macrophage colony-forming units in the CB graft may be a better marker of proliferative potential of HPCs although few studies have clearly documented an association with engraftment or survival (Iori et al, 2004; Yoo et al, 2007). Due to logistical, technical and economic reasons, the routine use of functional cell assessment remains difficult to perform in all collection centres. However, it is important to have functional assessment in the transplant centres after thawing the units because the results can have clinical implications in case of delayed engraftment.
Anti-HLA antibodies
Although there are conflicting data regarding the importance of HLA antibodies in the setting of CBT (Brunstein et al, 2011; Dahi et al, 2014), the presence of donor-specific anti-HLA antibodies (DSA) has been shown to adversely affect neutrophil and platelet engraftment in both ablative and RIC settings, using single or double unit CB grafts (Cutler et al, 2011; Ruggeri et al, 2013) and may also be associated with a higher TRM (Ruggeri et al, 2013) and inferior survival (Cutler et al, 2011). In light of these observations, all recipients should be screened for anti-HLA antibodies prior to transplant and CBUs for which the recipient has high levels of DSA should be avoided. At present, there are currently insufficient data to make specific recommendations for the level at which DSA's should be considered clinically relevant and what the optimal strategy (e.g. desensitization, plasma exchange or rituximab) should be used if such units cannot be avoided.
Other considerations
Thus far, data regarding direction of HLA mismatch, ABO compatibility, non-inherited maternal antigens and Killer-immunoglobulin receptor ligand matching are conflicting and therefore guidance on the routine incorporation of these factors into unit selection cannot be currently made.
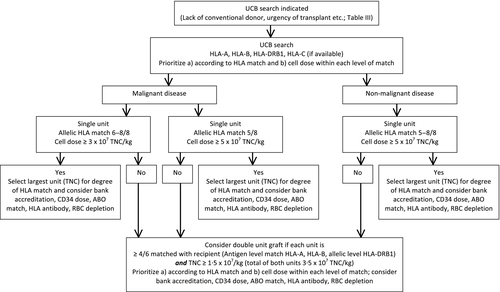
Algorithm for selection of individual UCB units
Table 1 and Fig 1 summarize recommendations for the selection of UCB units derived from these data.
| Initial selection of single cord blood units (CBU) should be based upon: | |
|
|
| (1) HLA-matching | |
| HLA-matching should be based upon allelic typing for HLA-A, -B, -C and -DRB1 for single UCBT | |
|
|
| (2) TNC and CD34+ cell dose | |
| Malignant disorders | |
| Nucleated cell dose |
At freezing, minimum TNC dose 3·0 × 107/kg, or After thawing, minimum TNC of 2·0–2·5 × 107/kg* |
| CD34+ cell dose** |
At freezing, 1·0–1·7 × 105/kg, or After thawing, around 1·0–1·2 × 105/kg |
| *If the infused TNC dose is 1·0–2·0 × 107/kg, the number of CD34+ cells or CFU-GM should be taken into consideration to predict the probability of neutrophil recovery and to discuss the possibility of a second transplant. If both cell doses are lower than recommended, a BM aspirate and chimerism analysis should be performed between day +20 and 28. The absence of engraftment indicates the need for a second transplant; preliminary data shows that haploidentical or double CBT should be considered | |
| **Due to variation in counting CD34+ cells, this recommendation should be taken with caution | |
| Colony-forming unit assay: When available, the CB bank should provide this information with that of the methodology used to set the assay up (semi-solid medium, cytokines, O2 tension) and score the colonies. This assay is important to evaluate the functional capacity of HPCs after thawing an aliquot or after thawing the product, however it is difficult to establish a generalized CFU-GM dose due to variations of colony setup and counting between centres. However if colonies are not growing the transplant physicians should consider a second transplant after day +30 | |
| Non-malignant disorders | |
| Nucleated cell dose |
At freezing, minimum cell dose 3·5 × 107/kg, or After thawing, minimum cell dose 3·0 × 107/kg |
| For patients with BM failure syndromes (aplastic anaemia or congenital bone marrow failure states) or haemoglobinopathies, the number of TNC at freezing should be greater than 5 × 107/kg | |
| CD34+ cell dose | At freezing or after thawing, >1·7 × 105/kg |
| Colony-forming unit assay: see above section on malignant disorders | |
| (3) Other considerations when selecting single CB units | |
| If many CBU meeting the criteria above are available, the following factors should also be considered: | |
|
|
| Additional criteria for double CBU selection | |
| When a single CBU unit contains insufficient cells (as specified above), double UCBT is recommended for the treatment of malignant disorders | |
| There are currently insufficient data to make recommendations for double UCBT in the treatment of non-malignant disorders | |
| (1) HLA-matching | |
|
|
| (2) Cell dose | |
| Nucleated cell dose |
At freezing, the sum of both CBUs >3·5 × 107/kg The minimum cell dose of each unit should be >1·5 × 107/kg |
| CD34+ cell dose | At freezing or after thawing, the sum of both CBUs > 1·8 × 105/kg |
| (3) ABO matching | |
| Recently, a Eurocord retrospective study of almost 1000 double UCBT recipients has shown an important association between ABO compatibility of two units with the patient on acute graft-versus-host disease, non-relapse mortality and overall survival. Thus, ABO compatibility between units and patients should be preferred over minor or major compatibility of one of the units between CB and patient (V Rocha on behalf of Eurocord, personal recommendation) | |
- CBU, cord blood units; HLA, human leucocyte antigen; TNC, total nucleated cell; (U)CBT, (umbilical) cord blood transplantation; CB, cord blood; BM, bone marrow; HPCs, haemopoietic progenitor cells; CFU-GM, granulocyte-macrophage colony-forming unit; NIMA, non-inherited maternal antigens; KIR, killer immunoglobulin receptor.
Donor selection algorithms
It is now possible to identify a potential donor for almost all patients in whom allogeneic HSCT is indicated. In addition to donor identification, many factors may impact on the optimal donor choice, including urgency of transplant, presence of donor-specific alloantibodies, clinical trial availability, centre expertise and funding constraints. Based on the UCBT data summarized above, international data comparing the clinical outcomes for different HSC donor source (Table 2) and the emerging outcome data for haploidentical transplantation, consensus recommendations for the role of cord blood in the overall donor selection strategy in the UK are summarized in Table 3.
| Disease | Patients (n) | Survival according to stem cell source | Organization (reference) | |||
|---|---|---|---|---|---|---|
| Matched related donor | Unrelated BM/PBSC donor | Unrelated CB donor | Haploidentical donor | |||
| (A) Paediatric recipients | ||||||
Mixed haematological disorders 2-year OS |
114 | – |
41% T-replete 56% T-deplete |
53% 52% |
– | Minnesota (Barker et al, 2001) |
Mixed haematological disorders 3-year OS |
64 | – | 57% | 59% | – | Montreal (Dalle et al, 2004) |
Acute leukaemia 2-year EFS |
541 | – |
43% T-replete 37% T-deplete |
31% | – | Eurocord (Rocha et al, 2001) |
Acute leukaemia 5-year EFS |
503 | – |
38% for MUD 37% for mMUD |
60% for matched 45% for 1 MM (high cell dose) 36% for 1 MM (high cell dose) 33% for 2 MM |
– | CIBMTR (Eapen et al, 2007) |
ALL 3-year EFS |
468 | – | – | 23% | 29% | Eurocord (Hough et al, 2007) |
ALL CR2 3-year LFS |
348 | 50% | 44% | 43% | – | IBMTR (Zhang et al, 2012) |
Primary immune deficiency 3-year OS |
444 | 71% | 59% | – | 42% | European (Antoine et al, 2003) |
Primary immune deficiency 5-year OS |
249 | – | – | 57% | 62% | Eurocord/SCETIDE (Fernandes et al, 2012) |
Wiscott Aldrich syndrome 5-year OS |
170 | 87% | 71% | – | 52% | IBMTR/NMDP (Filipovich et al, 2001) |
Hurler syndrome 5-year OS |
54 | 75% | – | – | 53% | Storage Disease Collaborative Group (Peters et al, 1998) |
Hurler syndrome 5-year EFS |
258 | 81% |
66% for MUD 41% for mMUD |
81% for 6/6 68% for 5/6 57% for 4/6 |
– | Eurocord/CIBMTR (Boelens et al, 2013) |
| (B) Adult recipients | ||||||
Haematological malignancy 2-year OS |
1377 | – |
49% for MUD 38% for mMUD |
39% | – | Japan (Tanaka et al, 2015) |
Haematological malignancy 5-year OS |
344 | – |
50% for MUD 39% for mMUD |
37% | – | Paris (Granier et al, 2015) |
| ALL | 165 | – | – | 36% | 13% | Eurocord (Rocha et al, 2005) |
AML 2-year EFS |
242 | 30% | 24% | |||
| ALL | 528 | – | – | 34% | 28% | Eurocord (Ruggeri et al, 2015) |
AML 2-year LFS |
918 | 38% | 32% | |||
AML 5-year OS |
651 | – |
49% MUD 33% for mMUD |
36% | – | SFGM-TC (Malard et al, 2015) |
AML 6-year OS |
414 | 47% |
54% MUD 51% for mMUD |
36% | – | Minnesota/Paris (Warlick et al, 2015) |
ALL CR 1 + 2 3-year OS |
802 | – |
44% for MUD 43% for mMUD |
44% | – | IBMTR (Marks et al, 2014) |
Acute leukaemia and CML 3-year OS |
166 | – |
57% for MUD 44% for mMUD |
73% | – | New York (Ponce et al, 2015) |
MDS 2-year OS |
631 | – | 49% | 30% | – | EBMT (Robin et al, 2015) |
Mature lymphoid malignancy 3-year OS |
645 | – | 49% | 56% | – | Eurocord (Rodrigues et al, 2014) |
Hodgkin lymphoma 2-year OS |
90 | 53% | 58% | – | 58% | Multicentre (Burroughs et al, 2008) |
- BM, bone marrow; PBSC, peripheral blood stem cells; CB, cord blood; OS, overall survival; EFS, event-free survival; LFS, luekaemia-free survival; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CML, chronic myeloid leukaemia; MDS, myelodysplastic syndrome; MUD, matched unrelated donor; mMUD, mis-matched unrelated donor; MM, mis-match; HSC, haemopoietic stem cells; CIBMTR, Center for International Blood and Marrow Transplant Research; IBMTR, International Bone Marrow Transplant Registry; EBMT, European Group for Blood and Marrow Transplantation; SCETIDE, Stem Cell Transplant for Immunodeficiencies in Europe; NMDP, National Marrow Donor Program; SFGM-TC, Société Française de Greffe de Moelle et de Thérapie Cellulaire.
| Choice | Family donor | Unrelated donor | Unrelated CB |
|---|---|---|---|
| Paediatric malignancy | |||
| 1st | MFD (BM, PBSC, CB) | – | – |
| 2nd | 9/10 FD | 10/10 | 6–8/8 (≥3 × 107/kg) |
| 9/10 | – | ||
| 3rd | – | – | 5/8 (≥5 × 107/kg) |
| 4th | Haploidentical FD | – | – |
| Adult malignancy | |||
| 1st | MFD (BM, PBSC) | – | – |
| 2nd | – | 10/10 | – |
| 3rd | – | 9/10 | 6–8/8 (≥3 × 107/kg) |
| – | 5/8 (≥5 × 107/kg) | ||
| 4th | Haploidentical FD (on trial) | – | – |
| Adult and paediatric bone marrow failure | |||
| 1st | MFD | – | – |
| 2nd | – | 10/10 | – |
| 9/10 | |||
| 3rd | Haploidentical FD | – | 5–8/8 (≥5 × 107/kg) |
| Paediatric immune deficiency and metabolic disorders | |||
| 1st | MFD (BM, PBSC, CB) | – | – |
| 2nd | – | 10/10 | 8/8 (≥5 × 107/kg) |
| 3rd | – | 9/10 | 6–7/8 (≥5 × 107/kg) |
| 4th | Haploidentical FD | – | 5/8 (≥5 × 107/kg) |
- MFD, matched family donor; BM, bone marrow; PBSC, peripheral blood stem cells; CB, cord blood; FD, family donor; TCD, T cell depletion; MM, mismatch.
- 1. A double umbilical cord blood graft can be substituted for a single unit graft in the table if each unit is 4–6/6 matched with the recipient and each unit has a total nucleated cell dose >1·5 × 107/kg.
- 2. The use of 8/10-matched unrelated donors is generally not recommended, but may be considered if no other suitable donor exists and: In adults (i) Campath is used for T cell depletion and (ii) the transplant centre has specific expertise in performing these procedures. In children 8/10 or 7/8 (excluding DQ) may be considered if nationally approved TCD methods are used.
- 3. Other factors may appropriately influence donor choice, e.g. urgency of transplant, remission status of the patient, specific disease group, cytomegalovirus serostatus of the donor and recipient, and carrier status of a family donor in the context of inherited disorders.
- 4. This guidance reflects the limited long-term follow data for newer haploidentical transplantation approaches compared to that available for sibling, unrelated donor and cord blood transplant. However, the early promising data for novel methods of haploidentical transplantation are noted and may necessitate review of these recommendations in due course.
The table is split into four categories; (i) paediatric malignancy, (ii) adult malignancy, (iii) BM failure (paediatric and adult) and (iv) paediatric immune deficiencies and metabolic disorders. For each disease group, the favourability of donor is indicated by ‘choice’ order (1st, 2nd, 3rd or 4th) such that 1st choice donors should be selected above 2nd, which should be selected above 3rd, which should be selected above 4th. All donors within a line of choice are considered to have expected equivalent clinical outcomes and can be reasonably selected if more than one donor within these categories is available for an individual patient. Where no single UCB unit of sufficient size is available a double cord blood graft can be considered.
The ranking system is based on HLA match and cell dose (for UCBT). HLA matching for family and unrelated donors is based on allelic typing for A, B, C, DRB1 and DQB1. HLA matching for single UCB units should now also be based on allelic typing for A, B, C and DRB1. A mismatch at the C locus is least preferable in the setting of CBT and should be avoided where possible.
At present, CBU selection for double cord transplants will continue to require 4–6/6 HLA matching, matching at antigen level for class I loci and allelic level for class II, due to insufficient data to justify selection of CBU for double UCBT using high resolution typing. There is now no requirement for inter-unit HLA matching. However, each unit must have a TNC >1·5 × 107/kg and the sum of the two CBU of TNC ≥3·5 × 107/kg or a total CD34 cell dose of ≥1·8 × 105/kg (Sanz et al, 2013) (Table 1).
Whilst the table summarizes a broad strategy for donor selection, there may be other factors that may, appropriately, influence donor choice. Urgency of transplant may preclude the choice of unrelated donor and necessitate a preference for UCB or a haploidentical donor. All patients for whom an alternative donor is needed should have an HLA antibody screen and donors selected accordingly. Disease status (homozygosity or heterozygosity) for sibling donors affected by inherited BM failure, immune deficiency and metabolic disorders should be taken into account in donor selection. For cytomegalovirus seropositive patients with malignancy, an unrelated donor UCBT may be preferable to a mismatched cytomegalovirus-negative unrelated volunteer donor (Shaw et al, 2014).
Conditioning regimens for UCBT
The rationale for the 2009 UK recommendations for RIC and MAC regimens for CBT was reviewed by Shaw et al (2009). Following the updates presented at the 2013 symposium and experience from the NCRI BSBMT clinical trials, the UK recommendations are now updated to also include the ‘Midi’ (mid-intensity) protocol developed by Juliet Barker (Flynn et al, 2007). This novel regimen consists of cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 10 mg/kg, and 400 cGy total body irradiation with ciclosporin and mycophenolate mofetil immunosuppression. In 30 patients (median age, 56 years; range, 18–69) with acute leukaemia or myelodysplasia, 97% of patients achieved neutrophil engraftment at a median of 26 d (range, 13–43) with a 93% platelet recovery by day 180. The incidence of acute GVHD (grades II–IV) was 67% at day 180, and chronic GVHD was 10% at 1 year. Overall, 2-year disease-free survival (DFS) was 60%. Comorbidity score, rather than age alone, significantly impacted on survival; 11 patients (median age, 55 years) with a Sorror score of 1 had a 2-year DFS of 82% compared with 62% in 9 patients (median age, 51 years) with a score of 2–3 and 40% in 11 patients (median age, 58 years) with a score of 4–5 (P = 0·13). In the UK, this regimen should be considered for older patients with good comorbidity scores instead of a conventional RIC regimen and as an alternative to MAC regimens in selected younger patients. Table 4 summarizes these ‘benchmark’ regimens.
| Intensity | Regimen |
|---|---|
| Reduced | Minneapolis |
| Fludarabine 40 mg/m2/d, days −6 to −2 (total dose 200 mg/m2) | |
| Cyclophosphamide 50 mg/kg, day −6 | |
| TBI 200 cGy (single fraction), day −1 | |
| Ciclosporin and mycophenolate mofetil GVHD prophylaxis | |
| Midi | Memorial Sloan Kettering |
| Fludarabine 30 mg/m2/d, days −6 to −2 (total dose 150 mg/m2) | |
| Cyclophosphamide 50 mg/kg, day −6 | |
| Thiotepa 5 mg/kg/d, days −5 to −4 (total dose 10 mg/kg) | |
| TBI 200 cGy/d, days −2 to −1 (total dose 400 cGy) | |
| Ciclosporin and mycophenolate mofetil GVHD prophylaxis | |
| Ablative | Minneapolis |
| Fludarabine 25 mg/m2/d, days −8 to −6 (total dose 75 mg/m2) | |
| Cyclophosphamide 60 mg/kg/d, days −7 to −6 (total dose 120 mg/kg) | |
| TBI 1320 cGy (in 8 fractions) (days −3 to day 0) | |
| Ciclosporin and mycophenolate mofetil GVHD prophylaxis | |
| Valencia | |
| Fludarabine 50 mg/m2/d, days −5 to −3 (total dose 150 mg/m2) | |
| Busulphan 3·2 mg/kg/d, days −5 to −3 (total dose 9·6 mg/kg) | |
| Thiotepa 5 mg/kg/d, days −7 to −6 (total dose 10 mg/kg) | |
| ATG 2 mg/kg, days −4 to −2 (total dose 6 mg/kg) | |
| Ciclosporin and mycophenolate mofetil GVHD prophylaxis | |
| Patients aged <2 years or AML/MDS/JMML patients <16 years | |
| Busulfan 3·2 mg/kg/d, days −9 to −6 (total dose 12·8 mg/kg) | |
| Cyclophosphamide 60 mg/kg/d, days −4 and −3 (total dose 120 mg/kg) | |
| Melphalan 140 mg/m2, day −2 | |
| Ciclosporin and mycophenolate mofetil GVHD prophylaxis | |
| Paediatric FTT regimen (non-TBI alternative ablative regimen) | |
| Fludarabine IV 30 mg/m2/d, days −7 to −3 (total dose 150 mg/m2) | |
| Treosulfan IV 14 g/m2/d, days −7 to −5 (total dose 42 mg/m2) | |
| Thiotepa 5 mg/kg iv twice a day, day −4 (total dose 10 mg/kg) | |
| Ciclosporin and mycophenolate mofetil GVHD prophylaxis |
- TBI, total body irradiation; GVHD, graft-versus-host disease; ATG, antithymocyte globulin; AML, acute myeloid leukaemia; MDS, myelodysplastic syndrome; JMML, juvenile myelomonocytic leukaemia.
Recommendations
These recommendations are based on currently available data summarized within this report. However, as new data continues to emerge, these guidelines will require regular review and updating.
- We recommend that UCB should be considered as an alternative source of HSC for transplantation for those patients without a suitably matched sibling or unrelated donor, defined as ‘standard’ or ‘clinical option’ transplants within the BSBMT transplant indications tables.
- We recommend that UCB transplants should be performed using the nationally recommended conditioning regimens, in JACIE [Joint Accreditation Committee-International Society for Cellular Therapy (ISCT) & European Group for Blood and Marrow Transplantation (EBMT)] accredited allogeneic transplant centres.
- We recommend that the nationally agreed donor selection algorithms should be used.
- The selection of specific UCB units is complex. We recommend that (i) the nationally agreed criteria for selecting individual UCB units should be used and (ii) consideration be given to establishing a UK UCB Selection Committee, reporting to the CBWG and BSBMT.
- We recommend on-going and regular review by the BSBMT of clinical outcomes of alternative donor HSCT in the UK.
- The development of an innovative UCBT trial portfolio in the UK is urgently required.
Conflict of interest
There are no relevant conflicts of interest.