Normal prothrombin times in the presence of therapeutic levels of apixaban – in-vivo experience from King's College Hospital
The recently published guidelines from the British Committee for Standards in Haematology (BCSH) on the measurement of non-coumarin anticoagulants and their effects on tests of haemostasis (Kitchen et al, 2014) provide a welcome steer for clinicians. The guidelines stipulate that, for apixaban, both the prothrombin time (PT) and activated partial thromboplastin time (APTT) are insensitive tests and patients may have normal coagulation times despite therapeutic concentrations. This recommendation is based on studies conducted on plasma samples spiked with apixaban (Barrett et al, 2010; Douxfils et al, 2013; Gouin-Thibault et al, 2014; Harenberg et al, 2014; Lowe et al, 2014). To complement this recommendation, we would like to share our experience from patients receiving apixaban at King's College Hospital.
Following the National Institute for Care Excellence's (NICE) approval for the use of apixaban for stroke prevention in the context of non-valvular AF in 2013, we have been prescribing apixaban for selected patients according to the local South London Cardiac and Stroke Network criteria (South London Cardiovascular & Stroke Network, 2012). Where clinically indicated (e.g. extremes of body weight, renal dysfunction or presence of interacting drugs), we measured the activity of the apixaban with the STA-liquid anti-Xa assay® (Diagnostica Stago, Asnières-sur-Seine, France), with appropriate calibrators and controls. We also measured PT with the STA-Neoplastine® CI Plus PT assay (Diagnostic Stago, Asnières-sur-Seine, France). Figure 1 illustrates the PT and corresponding apixaban concentrations for patients, where activity was measured.
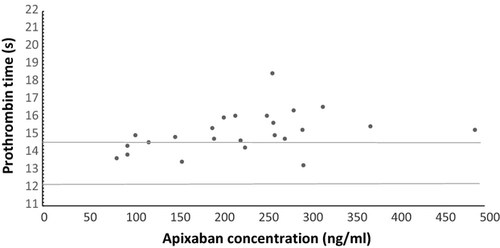
Our results demonstrate that the PT response for patients prescribed apixaban, is not as impressive as that observed with rivaroxaban previously, using the same reagent (Patel et al, 2013), and endorses the findings of published in-vitro work and the BCSH recommendation.
Why is this important? At King's, the current guidelines (http://www.kingsthrombosiscentre.org.uk/index.php/anticoagulation) for the management of bleeding for those patients receiving rivaroxaban recommends a PT screening test, in order to determine whether the presence of rivaroxaban may be contributing to bleeding. Our results suggest using the same strategy for patients prescribed apixaban would not be appropriate.
With increasing use of these direct-acting oral anticoagulants, it is apparent that a universal PT screening test, which detects the presence of all the direct-Xa inhibitors, does not currently exist. As such, laboratories will have to consider using an anti-Xa assay to determine the relative anticoagulant effect of apixaban, which will additionally provide a drug concentration.
Our results also highlight the potential risks associated with the use of screening tests for patients prescribed different direct Xa inhibitors; having one direct Xa inhibitor prolonging the PT and another not so, could be confusing. During the past year, we have had a few instances in our trust where patients have been admitted to hospital on a direct acting oral agent and have had an International Normalized Ratio or a coagulation screen ordered and inappropriately interpreted; this risk has previously been highlighted (Eby, 2013).
We once again endorse the recommendations made by the recent BCSH guidance on monitoring of direct oral anticoagulant therapy (Baglin et al, 2012), which stipulates that laboratories should be aware of the sensitivity of their own assays (PT and others) to each direct-acting oral anticoagulant. With an increasing repertoire of oral direct-acting anticoagulants, each of which have distinct effects on tests of haemostasis, education of non-specialist clinicians on how to interpret the results of coagulation tests is essential.
Conflicts of interests
The authors have no relevant conflicts of interest to declare.
Author contributions
JPP drew the patient samples. PBC analysed the samples. JC, LNR, RKP, RA and JPP co-wrote the letter.




