Expression of tumour necrosis factor-α and its receptors in Hodgkin lymphoma
In Hodgkin lymphoma (HL) patients, serum tumour necrosis factor-α (TNF-α, also termed TNF), TNF-α receptor 1 (TNFR1, also termed TNFRSF1B), and TNFR2 (also termed TNFRSF1B) levels are significantly higher than those in control subjects (Gruss et al, 1993; Salles et al, 1996; Metkar et al, 2000). Therefore, we evaluated the expression frequencies of TNF-α and TNFRs in HL and identified the cells from which Hodgkin and Reed/Sternberg (HRS) and lymphocyte predominant (LP)/popcorn cells originated in comparison with normal lymph node (LN) cells.
We immunohistochemically examined 26 specimens [23 classical HL (CHL) and three nodular lymphocyte predominant HL (NLPHL)] from the LNs of previously untreated HL patients diagnosed between 2003 and 2013 at the Osaka Medical College Hospital. As controls, 16 normal LN samples were obtained from biopsied specimens initially suspected of cancer metastasis.
Hodgkin lymphoma and normal specimens were fixed in 10% buffered formalin, embedded in paraffin, and cut into 4-μm thick sections. Immunohistological staining was performed with the EnVision system kit (DAKO, Glostrup, Denmark) using the following primary antibodies: CD3 (1:4 dilution; PS1 monoclonal; Nichirei, Tokyo, Japan), CD20 (1:400; SL26 monoclonal; Kyowa Medix, Tokyo, Japan), CD30 (1:50; Ber-H2 monoclonal; DAKO), TNF-α (1:50; 52B83 monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA), TNFR1 (1:50; H-271 polyclonal; Santa Cruz Biotechnology), TNFR2 (1:50; polyclonal; Enzo Life Sciences, Farmingdale, NY, USA), multiple myeloma oncogene 1 (MUM1, 1:20, MUM1p monoclonal; DAKO), and B-cell lymphoma 6 (BCL6, 1:20; PG-B6p monoclonal; DAKO). Epitope retrieval was performed via microwaving for the CD3, CD20, CD30, TNFR2, MUM1, and BCL6 antibodies and via trypsin digestion for the TNF-α and TNFR1 antibodies. In addition, we carried out CD30/MUM1 or BCL6, TNF-α/MUM1 or BCL6, TNFR1/MUM1 or BCL6, and TNFR2/MUM1 or BCL6 double staining with the EnVision G/2 system/AP (DAKO). For each antigen, reactivity in ≥20% of tumour cells was considered positive. In situ hybridization (ISH) was performed using the INFORM Epstein-Barr virus-encoded RNA (EBER) assay (Ventana Medical Systems, Inc., Tucson, AZ, USA).
The average age of the 23 CHL patients (18 males and five females) was 49 years (range, 16–88 years), with 10 having nodular sclerosis, 10 mixed cellularity and three lymphocyte-rich CHL type. The Ann Arbor classification system staging yielded three IA, six IIA, four IIB, two IIIA, six IIIB, one IVA and one IVB cases, and the International Prognostic Scoring (IPS) system yielded 1, 5, 7, 7 and 3 patients with scores of 0, 1, 2, 3 and 4, respectively. EBER was positive in eight cases (35%) and negative in 15 (65%). Three NLPHL patients were a 31-year-old female with stage IA and IPS 0, a 20-year-old male with stage IA and IPS 1 and a 39-year-old male with stage IA and IPS 1. All three NLPHL cases were negative for EBER.
All HRS cells were CD30-positive, CD20-negative and strongly positive for cytoplasmic TNF-α (Fig 1A), TNFR1 (Fig 1B), and TNFR2 (Fig 1C) staining, regardless of the disease subtype, stage, IPS score, and EBER status. In the double staining, the HRS cell nuclei were MUM1-positive (Fig 1D–G) but BCL6-negative. All LP/popcorn cells in the three cases were negative for CD30, and positive for CD20, TNF-α (Fig 1H), TNFR1 (Fig 1I) and TNFR2 (Fig 1J). Furthermore, the LP/popcorn cells were MUM1-negative but BCL6-positive or weakly BCL6-positive (Fig 1K–N). In both CHL and NLPH, only a few normal lymphocytes in the background stained weakly for TNF-α, TNFR1 and TNFR2. In normal LN, immunoblasts, which are large CD20-positive cells with conspicuous nucleoli, are located in the peripheral germinal centre (GC) rim (Hu et al, 2013) and migrate from the peripheral GC to outside the marginal and T-cell zones. Some peripheral GC rim immunoblasts were CD30-positive (Fig 2A), whereas others were CD30-negative (Fig 2B). In contrast, nearly all marginal and T-cell zone immunoblasts were CD30-positive (Fig 2C). In addition, nearly all peripheral GC rim, marginal zone and T-cell zone immunoblasts were TNF-α- (Fig 2D, E), TNFR1- (Fig 2F, G) and TNFR2-positive (Fig 2H, I).
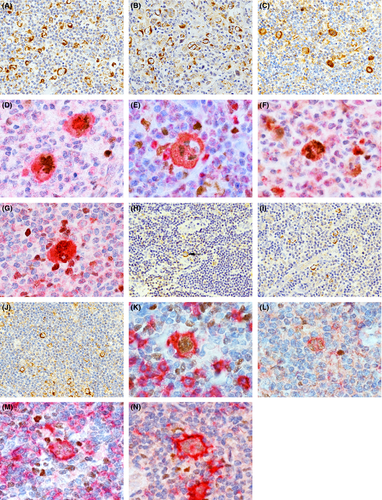
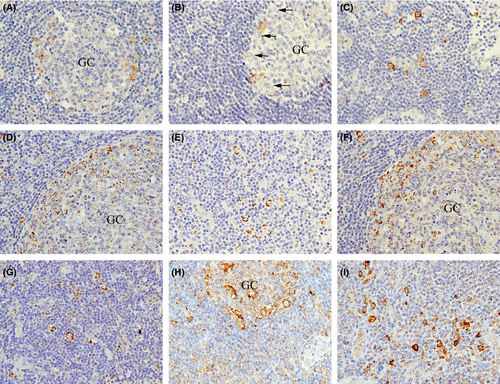
In immunoblasts, CD30 expression is induced by exposure to several activating agents, such as phytoheamagglutinin, bacteria, human T leukaemia virus or EBV, and plays an important role in promoting malignant cell growth and survival (Stein et al, 1985; Deutsch et al, 2011). CD20 is a pan-B-cell immunophenotypic marker expressed on normal and neoplastic B lymphocytes. However, CD20 and CD30 double-positive B-cell lymphomas are rare, suggesting that CD20 expression may be downregulated by CD30 expression (Li et al, 2012). In HRS cells, CD30 expression probably affects the lack of CD20 expression, and downregulated CD20 expression may be caused by defective CD20 transcription in the neoplastic event, similar to the downregulated immunoglobulin production consequent to defective immunoglobulin transcription (Marafioti et al, 2000).
As TNF-α, TNFR1, and TNFR2 are expressed on HRS and LP/popcorn cells, they may play roles in reactive lymphocyte infiltration and tumourigenesis via a TNF-α-mediated autocrine mechanism. HRS cells exhibit low or no expression of GC B-cell proteins, such as BCL6 but nearly constitutive expression of the late GC/post-GC B-cell protein MUM1 (Valsami et al, 2007). In contrast, LP/popcorn cells express BCL6, but MUM1 expression is rare (Herbeck et al, 2011). Our double-staining results supported these findings. Although a further evaluation with a larger sample size is needed, the present study may introduce the hypothesis that HRS cells in CHL originate from CD30-, TNF-α-, TNFR1- and TNFR2-positive activated immunoblasts in the late GC, marginal zone and T-cell zone (post-GC) in response to EBV or another unknown infection and that LP/popcorn cells in NHPHL may originate from CD30-negative but TNF-α-, TNFR1- and TNFR2-positive immunoblasts in the GC.
Acknowledgements
This study was supported by a grant from the Osaka Community Foundation. Shoko Nakayama conceived and designed the study, analysed the data and wrote the paper. Shoko Nakayama and Motomu Tsuji performed immunostaining. All authors read and approved the final manuscript.




