TET2 gene sequencing may be helpful for myeloproliferative neoplasm diagnosis
Myeloproliferative neoplasms (MPNs) are chronic malignancies characterized by the deregulation of haematopoietic progenitor functions. During disease evolution, these patients are prone to develop severe arterial or venous thrombosis, such as myocardial infarction or splanchnic vein thrombosis (SVT), and transformation to myelofibrosis or acute leukemia may occur. The discovery of the JAK2 V617F mutation in the majority of MPN patients (James et al, 2005) has changed the diagnostic algorithm of MPNs and this molecular marker is now included in the most recent World Health Organization (WHO) classification for Polycythaemia Vera (PV), Essential Thrombocythaemia (ET) and Primary Myelofibrosis (PMF) (Tefferi & Vardiman, 2008). However, many patients with ET and PMF (and a few with PV) do not carry the JAK2 V617F mutation. Other mutations have been identified, such as the JAK2 exon 12 mutations in 1% of PV and the MPL gene mutations in 5–10% of ET or PMF. In most cases these mutations are exclusive. Several other mutations have also been identified in MPNs that do not affect signalling pathways regulators and are not exclusive of the previously identified mutations. These mutations are also found in a significant proportion of other myeloid malignancies. The most prevalent mutations in this category are in the TET2 gene (Delhommeau et al, 2009), which are present in 16% of PV, 5% of ET and 17% of PMF (Tefferi et al, 2009), and have also been reported together with JAK2 V617F in SVT patients (Colaizzo et al, 2010). As the last WHO classification stated that one major criterion for MPN diagnosis was the presence of a molecular marker such as JAK2 V617F or equivalent, an unsolved question which gene alterations that may be included in this classification in addition to JAK2 and MPL mutations. The present study assessed the role of TET2 mutations in the diagnosis of different MPN subtypes when JAK2 and MPL mutations are missing.
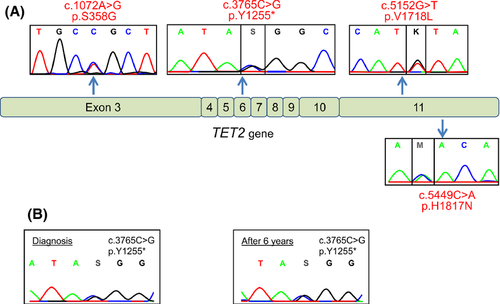
We studied a cohort of 50 patients suspected of MPN but negative for both JAK2 and MPL mutations. Diagnosis was PV (n = 10), ET (n = 12), PMF (n = 5) or suspicion of latent MPN revealed by SVT (n = 23) among which six presented with a Budd-Chiari Syndrom and 17 with Portal Vein thrombosis. The detection of JAK2 V617F, JAK2 exon 12 or MPL mutations was performed on DNA extracted from peripheral blood leucocytes as previously described (Kouroupi et al, 2012). The entire coding sequence of TET2 (11 exons) was analysed by direct Sanger sequencing. Previously reported TET2 gene mutations were found in 5 (10%) out of the 50 patients analysed. Four missense and 1 nonsense mutations (Fig 1A) were detected in two PV, one ET and two SVT patients (Table 1). The presence of a TET2 mutation reinforced the diagnosis of PV and ET (already made using the WHO minor criteria) in Patients 44, 46 and 49. Of the two TET2 mutated patients with PV, one secondarily evolved to myelofibrosis 6 years after PV diagnosis (Patient 46). A retrospective analysis demonstrated the presence of the TET2 mutation with the same mutant allele burden since the diagnosis of PV (Fig 1B). TET2 mutations have been reported to be a pre-JAK2 event, preceding the appearance of a MPN phenotype (Delhommeau et al, 2009), but their later acquisition, at the time of transformation to myelofibrosis or leukaemia, has also been reported (Schaub et al, 2010). In this study, the presence of the TET2 mutation was unrelated to the transformation as, in the only patient who developed myelofibrosis, the mutation was already present at PV diagnosis and there was no evidence for a progression of the TET2 mutant clone with time (Fig 1B). The four other patients had not transformed to myelofibrosis after a median follow-up of 86 months.
| Patient | Age (years) | Sex | Diagnosis | TET2 mutation | Thrombosis | Evolution | Follow-up (years) |
|---|---|---|---|---|---|---|---|
| 6 | 47 | M | SVT | c.5152G>T, p.V1718L | Portal vein | No overt MPN | 6 |
| 7 | 34 | M | SVT | c.5449C>A, p.H1817N | Portal vein | No overt MPN | 8 |
| 44 | 38 | F | ET | c.5152G>T, p.V1718L | None | None | 5 |
| 46 | 62 | M | PV | c.3765C>G, p.Y1255* | Arterial (necrosis of foot sole) | Myelofibrosis | 8 |
| 49 | 59 | F | PV | c.1072A>G, p.S358G | None | None | 9 |
- M, male; F, female; SVT, splanchnic vein thrombosis; PV, polycythaemia vera; ET, essential thrombocythaemia; MPN, myeloprolifrative neoplasm.
- , Stop codon.
In patients with SVT without identified risk factor for thrombosis, an underlying MPN must be ruled out even when the blood cell counts are within the normal ranges. MPN diagnosis in such patients may modify the therapeutic options, as cytoreductive therapy may be considered to avoid thrombotic recurrences. Several biological investigations may be conducted to diagnose MPN, among which an elevated red cell mass, typical MPN features on bone marrow biopsy and the identification of a molecular marker are strongest evidences. In our study, two out of 23 patients presenting with SVT but no overt MPN had a TET2 mutation. Both patients had portal vein thrombosis. Patient 7 presented with a normal haematocrit but elevated red cell mass and normal circulating erythropoietin levels. No overt MPN appeared after 6 and 8 years of follow-up for these SVT patients harbouring TET2 mutations, which is frequently observed in JAK2-mutated SVT patients as well. Among the 21 SVT patients without TET2 mutation an MPN was suspected based on bone marrow examination and/or red cell mass measurement but none of them developed an overt MPN after a median follow-up of 54 months.
To date, many mutated genes have been reported in MPN patients that can be classified into four groups: (i) mutations altering signalling pathways (JAK2, MPL) (ii) mutations altering the spliceosome (SF3B1, SRSF2) (iii) mutations altering epigenetics (TET2, EZH2, ASXL1, SUZ12…) (iv) mutations altering oncogenes (P53, N-RAS, K-RAS…). Only the mutations from Group 1 have proven to be able to confer an MPN phenotype in animal models or in vitro studies, but not TET2 mutations (Moran-Crusio et al, 2011). However, the TET2 mutated patients presented in this study have diagnostic features and clinical evolution very similar to patients bearing JAK2 or MPL mutations, suggesting that other molecular alterations may be responsible for the MPN phenotype in addition to TET2 mutations. However, screening for additional mutations as a hallmark of a clonal haematopoiesis has been proposed to help in MPN diagnosis (Schnittger et al, 2012), with TET2 as the first target because of its relatively high frequency of mutation. Our study suggests that the detection of TET2 mutation may be useful in a small number of patients to prove the diagnosis of MPN. However, it would be useful to analyse several other genes recurrently found mutated in MPN such as ASXL1 or EZH2 in order to increase the number of patients that would benefit from such diagnostic approaches.
Acknowledgements
We would like to thank Pr Elisabeth Tournier-Lasserve, Dr Florence Riant and Dr Laura Llopis for their precious help in TET2 sequencing.
Authorship contributions
EV performed the molecular analyses, AA analysed clinical data, CC provided samples, BC and JJK designed the study and wrote the manuscript, which was reviewed by all the authors.
Conflict of interest
Authors have no conflict of interest to disclose.




