Milatuzumab and veltuzumab induce apoptosis through JNK signalling in an NF-κB dependent human transformed follicular lymphoma cell line
Follicular lymphoma (FL) remains incurable with current standard therapy, despite advances in targeted agents. Improved therapy for FL and transformed FL (TxFL) would be aided by well-characterized experimental models. As the characteristic FL translocation t(14;18) de-regulates BCL2 and blocks apoptosis, rational strategies targeting apoptosis resistance are appealing. Much pre-clinical testing, however, is in model systems representing more aggressive lymphoma as few well-characterized models of FL or TxFL exist. We previously established the t(14:18)+ FL model cell line WSU-FSCCL (Mohammad et al, 1993), and demonstrated its response to targeted therapeutics (Smith et al, 2004). We now characterize t(14:18)+ cell lines from a patient with TxFL in vitro and as a severe combined immunodeficiency (SCID)-mouse xenograft and demonstrate utility for pre-clinical evaluation of targeted therapies.
FC-TxFL-1 and FC-TxFL-2 were established from pleural fluid obtained 3 months apart from a 58-year-old man who had presented a year earlier with advanced stage FL. At the initial sampling, source of FC-TxFL-1, he had stable nodal disease following two cycles of CVP (cyclophosphamide, vincristine, prednisolone) and no other evidence of transformation aside from the pleural effusion, which revealed predominantly small T lymphocytes with CD4+/CD8+ ratio of ~3, and ~5% small B cells which were clonal, without evidence of large cell transformation. By the second thoracentesis (source of FC-TxFL-2), he had aggressive disease and this sample contained mostly large transformed B cells. Thus, the transformed cell was present at least 3 months before becoming clinically evident.
Ficoll-Hypaque separated lymphocytes were suspended in RPMI-1640 medium containing 15% heat-inactivated fetal bovine serum (HI-FBS), 25 μg/ml insulin, 1 mmol/l Na-pyruvate, 1 μmol/l β-mercaptoethanol, 1% L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. After 30 (FC-TxFL-1) and 16 (FC-TxFL-2) passages, media was simplified to RPMI-1640 medium containing 10% HI-FBS, glutamine, penicillin and streptomycin, grown at 37°C in humidified 5% CO2. Cytogenetic analysis was performed by the Fox Chase Cancer Center (FCCC) Cytogenetic Core Facility as before (Smith et al, 2013). Epstein-Barr virus (EBV) detection was by DNA-polymerase chain reaction (PCR) with Bam H1W promoter site primers. Immunophenotyping used the standard clinical panel of FCCC Flow Cytometry laboratory. Cells stained per manufacturer's recommended methods with the indicated direct fluorescently labelled antibody were analysed on Becton Dickinson (BD) FACS system (BD BioSciences, Mountainview, CA). RNA-PCR was as previously described (Mohammad et al, 1993).
FC-TxFL-2 cells (1.0 × 106/0.2 ml in 96-well plate) were cultured in the presence or absence of 5 μg of the indicated antibody ± 25 μg goat anti-human IgG antibody (GAH, Jackson Immunoresearch Laboratory, West Grove, PA, USA) for 30 min, 4 or 24 h. Antibodies such as rituximab often must be cross-linked in vitro to affect cells, achieved passively by coating the antibody on the plate or actively using GAH. The monoclonal antibodies milatuzumab (Stein et al, 2007) (humanized IgG1 anti-CD74 antibody LL1), epratuzumab (Leonard & Goldenberg, 2007) (humanized anti-CD22 antibody hLL-2) and veltuzumab (Goldenberg et al, 2009) (humanized anti-CD20, hA20) were supplied by Immunomedics, Inc. (Morris Plains, NJ, USA). Rituximab was a commercial product (Roche-Genentech, South San Francisco, CA, USA). Where indicated, cells were pretreated with 10 μmol/l NF-κB inhibitor (wedelolactone, IKK inhibitor II) or 15 μmol/l JNK inhibitor (SP600125) (BIOMOL, Plymouth Meeting, PA, USA) for 60 min. Annexin V apoptosis assay (Pharmingen, BD Biosciences) was as before (Smith et al, 2005).
Immunoblotting and detection were as before (Smith et al, 2005), except that phosphatase inhibitor (phosStop, Roche Applied Sciences, Mannheim, Germany) was added and separation was on NuPAGE 4-12% Bis-Tris Gel (Invitrogen, Carlsbad, CA, USA) before transfer to Immobilon-P membrane (Millipore, Billerica, MA, USA). Membrane was blocked for 1 h, then incubated at 4°C overnight with primary antibody (1:1000 dilution) to cleaved caspase-3, β-actin, phosphorylated (p)-JNK or p-NF-κB (Cell Signaling Technology, Danvers, MA, USA).
Female CB17 SCID mice were maintained in the FCCC Laboratory Animal Facility under an approved protocol. Four- to eight-week-old mice, injected intraperitoneally (ip) with 1 × 107 FC-TxFL-2 cells were observed daily and euthanized when ill. Lymphoma involving diffuse adenopathy, splenomegaly, ascites and infiltration of liver and marrow developed in untreated mice at 4–5 weeks. As discrete tumors did not consistently develop with any injection route, survival was the efficacy endpoint.
Both transformed follicular lymphoma B cell lines FC-TxFL-1 and -2 are EBV-negative and reflect the patient's lymphoma phenotypically [expression of CD10, CD19, CD20, FMC7, CD22, CD38, CD79a, and surface IgM kappa; absent CD5, CD23, or T-lymphoid, myeloid and monocyte antigens]; karyotypically [46,XY,add (1)(p36.3), del (2)(p23), t(3:6) (p21;p21.3), -10, add(10)(q26),der(11), t(1;11) (q12;q23), ins (12), t(14;18)(q32;q21),+mar1]; and molecularly [BCL2 major breakpoint region juxtaposed with immunoglobulin (Ig) heavy chain joining region 6 (IGHJ6). By RNA-PCR, the predominant translocation transcript is BCL2-IGHJ6 spliced to IGHM.
For therapy, we focused on FC-TxFL-2. After 48-h incubation with antibody plus GAH, FC-TxFL-2 cell count was reduced to ~30% of control with GAH-cross-linked veltuzumab or milatuzumab, but to only ~65% with epratuzumab. Similarly, apoptosis (annexin V) indicated these cells were most sensitive to cross-linked milatuzumab, less to cross-linked veltuzumab and not to cross-linked epratuzumab (not shown).
FC-TxFL-2 cells have constitutive levels of p-JNK and p-NF-κB. Rituximab alone did not alter these levels, however, rituximab cross-linked with GAH increased p-JNK within 30 min. This persisted at 4 h and returned to baseline by 24 h (Fig. 1A). There were no significant changes in p-NF-κB (not shown). Cross-linked veltuzumab yielded alterations similar to rituximab (Fig. 1B, lane 8). Cross-linked milatuzumab also induced p-JNK, but with a different time-course, not until 24 h (Fig. 1B, lane 4). Epratuzumab had minimal effects.
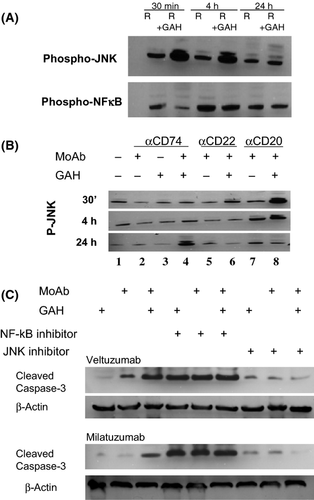
We used inhibitors to investigate whether these signal pathways were critical to the induction of apoptosis, assessed by caspase 3 cleavage. Apoptosis is induced by cross-linked veltuzumab or milatuzumab. While JNK inhibition itself had no effect, JNK inhibition blocked antibody-induced apoptosis. JNK signalling, activated by MAP kinase, can affect both intrinsic and extrinsic apoptotic pathways (Dhanasekaran & Reddy, 2008) and warrants further evaluation in FL. In contrast to JNK, NF-κB inhibition alone induced apoptosis, suggesting that tonic NF-κB signalling (Jost & Ruland, 2007) is required for FC-TxFL-2 survival. NF-κB has not been considered critical to germinal centre-derived lymphoma, including FL (Lam et al, 2005).
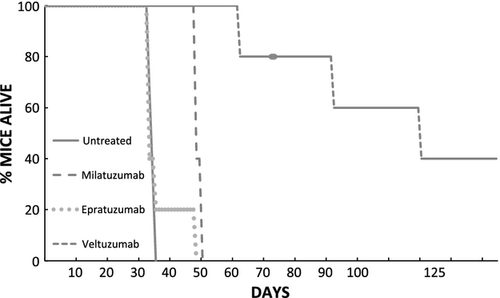
Single injections of either veltuzumab or milatuzumab, but not epratuzumab prolonged survival of FC-TxFL-2 bearing mice (Fig. 2). As with WSU-FSCCL, antisense oligonucleotide down-regulation of BCL2 enhanced antibody killing (not shown).
FC-TxFL-2 is a transformed FL model that is: (i) induced by anti-CD20 and anti-CD74 monoclonal antibodies to undergo apoptosis through JNK signalling, (ii) dependent on tonic NF-κB signalling for survival signals, and (iii) an in vitro and in vivo model to investigate targeted therapeutics in TxFL.
Acknowledgements
Supported in part by NCI PO1 CA103985 (Program PI: D.M. Goldenberg, Center for Molecular Medicine and Immunology, Belleville, NJ), and by NCI P30 CA006927, utilizing the Fox Chase Cancer Center Core Facilities in Genomics, Flow Cytometry and Laboratory Animal Facility. Additional support was from the Janice Charach Epstein Research Fund and Lester Smith Research Fund of Fox Chase Cancer Center.
FJ and IJ performed the research. MRS with assistance from IJ designed the research study. MRS and IJ analysed the data. MRS wrote the paper.
Conflicts of Interest
None to declare.




