Hepcidin decreases over the first year of life in healthy African infants
Iron metabolism is dynamic during infancy, with iron requirements 10-fold higher than in adults, because of rapid infant growth (Tolentino & Friedman, 2007). Hepcidin, a 25-amino acid peptide hormone secreted by hepatocytes, is the major regulator of iron metabolism (Ganz et al, 2008; Galesloot et al, 2011). Hepcidin synthesis is stimulated by elevated plasma iron, infection and/or inflammation, resulting in decreased availability of circulating iron; synthesis is decreased during demand for increased serum iron, such as hypoxia and iron deficiency (Galesloot et al, 2011).
Infants are at high risk of anaemia, particularly in developing countries; however, little is known about hepcidin levels during the first year of life. We set out to characterize normal hepcidin values in healthy, non-anaemic, Zimbabwean infants.
This study utilized cryopreserved plasma samples from ZVITAMBO (Zimbabwe Vitamin A for Mothers and Babies), a randomized controlled trial of maternal and neonatal vitamin A supplementation (Humphrey et al, 2006). Mother-infant pairs (n = 14 110) were recruited within 96 h of delivery in Harare, Zimbabwe, between 1997 and 2000. Maternal-infant pairs were eligible if neither had an acute life-threatening condition and the infant was a singleton with birth weight >1·5 kg. Follow-up was conducted at 6 weeks, 3 months and every 3 months thereafter, until the child was 12–24 months old. Haemoglobin was measured by haemoglobinometer (HemoCue, Mission Viejo, CA, USA) in a 34% subsample of infants (Miller et al, 2003). Written informed consent was obtained from mothers at recruitment. The original ZVITAMBO trial and this sub-study were approved by the Medical Research Council of Zimbabwe and Johns Hopkins Bloomberg School of Public Health.
Human immunodeficiency virus-negative infants were selected at 3, 6 and 12 months of age based on the following criteria: gestational age >37 weeks; birth weight >2·5 kg; absence of iron-deficiency anaemia (defined as haemoglobin >105 g/l at 3 and 6 months, or >100 g/l at 12 months; serum ferritin >12 μg/l; and soluble transferrin receptor (sTfR) <8·3 mg/l); absence of inflammation (defined as alpha-1 acid glycoprotein (AGP) <1 g/l and C-reactive protein (CRP) < 5 mg/l); absence of acute illness (defined as diarrhoea or fever in the preceding week, or measles in the preceding 3 months). This yielded a sample size of 139 infants with three cross-sectional groups at 3 months (n = 60), 6 months (n = 47) and 12 months (n = 40), with 132 infants contributing data at one time point, six infants contributing data at two time points and one infant contributing data at all three time points. Anaemia criteria were modified from World Health Organization guidelines because of the growing literature supporting lower haemoglobin cut-offs in infancy (Domellof et al, 2002; Miller et al, 2003).
Plasma sTFR and ferritin were measured by enzyme immunoassay (Ramco Laboratories Inc, Houston, TX, USA). Plasma AGP and CRP were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems Inc, Minneapolis, MN, USA). Plasma hepcidin was measured in triplicate, using a 1:4 dilution in peptide-cleared human serum, by competition ELISA (S-1337; Bachem, San Carlos, CA, USA) with detection range 0·02–25 ng/ml. Samples with readings outside the linear region of the curve were re-run at alternative dilutions. The intra-assay coefficient of variation (CV) was mean 6·3% (range 5·7–6·9%), and inter-assay CV was 6·3%. Hepcidin values below the detection limit (0·02 ng/ml) were imputed using limit of detection/√2, thereby assigning a value of 0·014 ng/ml to avoid zero values dropping out after log transformation. Hepcidin consensus values (hepcon1) were calculated using the algorithm developed by Kroot et al (2012) to enable interpretation across studies using different assays (Kroot et al, 2012). All statistical analyses were performed with stata version 12 (StataCorp, College Station, TX, USA).
Infant baseline characteristics are shown in Table 1. Mean (standard deviation; SD) haemoglobin in infants aged 3 months (n = 60), 6 months (n = 47) and 12 months (n = 40) was 117·9 (13·4), 118·0 (9·4) and 117·1 (11·8) g/l, respectively. Ferritin levels were lower in the older age groups (P < 0·001; Kruskal–Wallis).
| Baseline characteristics | 3-month-old infants | 6-month-old infants | 12-month-old infants |
|---|---|---|---|
| (N = 60) | (N = 47) | (N = 40) | |
| Infant characteristics | |||
| Male sexa | 46·7 (28) | 46·8 (22) | 52·5 (21) |
| Gestational age, weeksb | 39·6 (1·2) | 39·7 (1·2) | 39·5 (1·2) |
| Birth weight, kgb | 3·105 (0·376) | 3·186 (0·359) | 3·23 (0·459) |
| Apgar score at 5 minc | 10 (10, 10) | 10 (9, 10) | 10 (10, 10) |
| Haemoglobin at blood sampling, g/lb | 117·9 (13·4) | 118·0 (9·4) | 117·1 (11·8) |
| Ferritin at blood sampling, μg/lc | 44·1 (27·9, 103·6) | 25·2 (17·6, 42·7) | 22·2 (15·9, 32·9) |
| sTfR at blood sampling, mg/lc | 5·71 (4·61, 6·67) | 5·91 (4·55, 6·44) | 6·00 (3·94, 6·74) |
| AGP at blood sampling, g/lc | 0·35 (0·27, 0·48) | 0·51 (0·30, 0·61) | 0·49 (0·39, 0·63) |
| CRP at blood sampling, mg/lc | 0·21 (0·11, 0·91) | 0·32 (0·13, 1·17) | 0·54 (0·18, 1·20) |
| Hepcidin at blood sampling, ng/mlc | 9·70 (2·50, 19·25) | 4·50 (0·49, 7·32) | 1·90 (0·73, 6·17) |
| Hepcidin consensus valuesd at blood samplingc | 6·20 (0·59, 13·65) | 2·15 (−0·98, 4·35) | 0·12 (−0·80, 3·45) |
| WAZ at birthb | −0·42 (0·79) | −0·24 (0·75) | −0·18 (0 .95) |
| WAZ at blood samplingb | −0·07 (0·91) | −0·07 (1·09) | −0·39 (1·23) |
| LAZ at birthb | 0·13 (0·90) | 0·11 (0·71) | 0·32 (1·44) |
| LAZ at blood samplingb | −0·32 (0·83) | −0·36 (1·23) | −0·84 (1·36) |
| Neonatal vitamin A treatmenta,e | 51·7 (31) | 55·3 (26) | 52·5 (21) |
| Maternal vitamin A treatmenta,e | 55·0 (33) | 48·9 (23) | 50·0 (20) |
| Maternal and household Characteristics | |||
| Maternal Education, yearsc | 11 (9, 11) | 11 (9, 11) | 11 (9, 11) |
| Mothers Employeda | 16·7 (10) | 10·9 (5) | 15·0 (6) |
| Maternal MUAC, cmb | 26·4 (2·8) | 25·9 (2·4) | 26·6 (2·7) |
| Household income per month, US$c | 1220 (801, 1693·5) | 1220 (855, 1830) | 1221 (879, 2103) |
- a Values are % (n).
- b Values are mean (standard deviation).
- c Values are median (interquartile range).
- d Hepcidin consensus values were calculated using the algorithm Y = −1·36 + 0·78*hepcidin (Kroot et al, 2012).
- e In the ZVITAMBO trial, mother-infant pairs were randomized within 96 h of birth to one of four treatment groups (Aa, Ap, Pa, Pp), where ‘A’ was maternal vitamin A supplementation (400 000 iu), ‘P’ was maternal placebo, ‘a’ was infant vitamin A supplementation (50 000 iu) and ‘p’ was infant placebo. Full details of the trial have been published elsewhere (Humphrey et al, 2006).
- sTfR, soluble transferrin receptor; AGP, alpha-1 acid glycoprotein; CRP, C-reactive protein; WAZ, weight-for-age Z-score; LAZ, length-for-age Z-score; MUAC, Mid-upper arm circumference, SD, standard deviation, IQR, interquartile range.
Hepcidin was higher at 3 months of age {median 9·70 ng/ml [interquartile range (IQR) 2·50, 19·25]}, than when aged 6 months [4·50 ng/ml (IQR 0·49, 7·32)] and 12 months [1·90 ng/ml (IQR 0·73, 6·17)] (P < 0·001; Kruskal–Wallis; Table 1). Hepcidin was undetectable in one infant at 6 months. Hepcon1 values were median (IQR) 6·20 (0·59, 13·65), 2·15 (−0·98, 4·35) and 0·12 (−0·80, 3·45) in infants aged 3, 6 and 12 months, respectively. Hepcidin was higher in girls than boys at each age, but sex differences were not significant in univariate regression analysis and after adjustment for age (Fig 1).
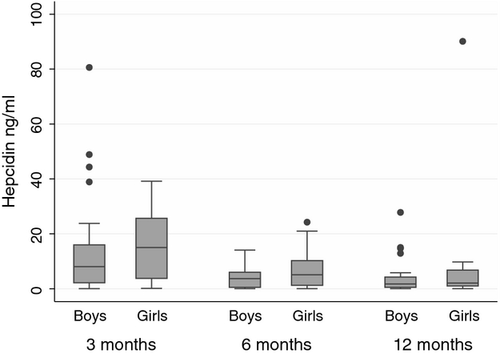
Iron metabolism is dynamic in infancy, with haemoglobin and ferritin falling after birth as breakdown of fetal erythrocytes exceeds formation of new erythrocytes (Dallman, 1987). Anaemia prevalence therefore increases throughout infancy as endogenous supplies of iron become depleted and dietary intake is insufficient to meet demands. We show for the first time in healthy, non-anaemic infants that hepcidin decreases throughout infancy, with most of the drop occurring between the ages of 3 and 6 months. This is presumably a physiological response to mobilize iron stores and absorb more dietary iron, thus preventing anaemia. Comparison of infant and adult values is complicated by differences between hepcidin assays used; we therefore report hepcidin consensus values to facilitate comparisons. Konopásek et al (2011) reported mean (SD) hepcidin levels of 15·43 (2·11) ng/ml in healthy adults, using the same assay. Infant values therefore appear lower than those reported in adults, supporting the higher metabolic need for iron in infancy compared to adulthood. Sex and age differences in hepcidin have been observed in adults (Galesloot et al, 2011); however, the higher values we observed in girls were not significant in our cohort, perhaps because of small numbers. Boys have been observed to be more vulnerable to anaemia (Thorisdottir et al, 2011); lower hepcidin in boys might therefore be a physiological response to inherently lower iron stores (Thorisdottir et al, 2011). Further studies are needed to determine hepcidin levels in anaemic infants to better understand the pathophysiology underlying this highly prevalent public health problem.
Acknowledgements
This work forms part of the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) project. Members of the SHINE project team are: G Mangwadu, C Chasokela, A Chigumira (Zimbabwe Ministry of Health and Child Welfare); J Humphrey, M Mbuya, A J Prendergast, F Van der Keilen, R Ntozini, N Tavengwa, P Rambanepasi, K Mutasa, F Majo, V Sauramba, P Hwena, B Chasekwa, B Mutasa, S Rukobo, D Maunze, J Mushonga (Zvitambo Institute for Maternal Child Health Research, Harare, Zimbabwe); J Humphrey, L Moulton (Johns Hopkins Bloomberg School of Public Health, Baltimore MD); R Stoltzfus (Cornell University, Ithaca, NY); A J Prendergast (Queen Mary, University of London, UK); J Tielsch (George Washington University, Washington, DC); G Mangwadu and J Humphrey are the co-principal investigators of SHINE.
Funding
The SHINE project is supported by the Bill and Melinda Gates Foundation (OPP1021542); Department for International Development, UK; National Institutes of Child Health and Human Development (R01HD060338-01); Wellcome Trust (093768/Z/10/Z); and The Swiss Agency for Development and Cooperation (Contract no. 81016727).
Author contributions
T.G.M., R.J.S., and A.J.P. designed the research. T.G.M. and S.R. performed experiments. T.G.M., R.J.S., L.H.M. and A.J.P., analysed data. T.G.M. and A.J.P. wrote the manuscript. T.G.M., R.J.S., S.R., L.H.M. and A.J.P. edited the manuscript.
Conflict-of-interest disclosure
None of the authors have any conflicts of interest to declare.




