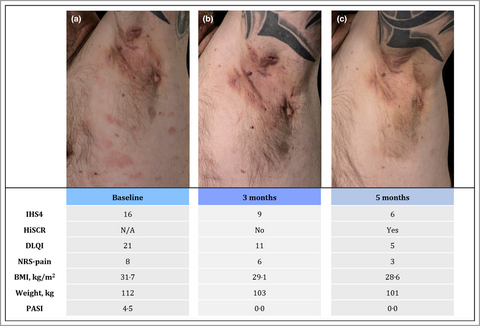
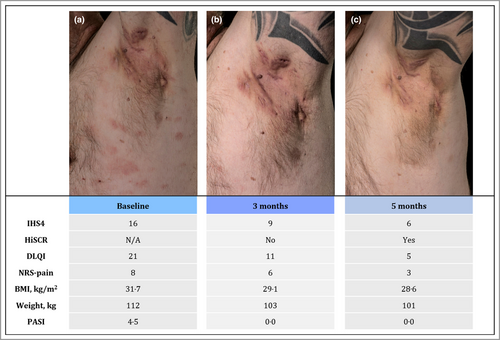
Considerable improvement in hidradenitis suppurativa with oral roflumilast therapy
Funding sources: none.
Conflicts of interest: the authors declare they have no conflicts of interest.
Data availability: the data that support the findings of this study are available on request from the corresponding author.
Hidradenitis suppurativa (HS) is a chronic, inflammatory disease characterized by recurrent nodules, tunnels and excessive scarring in predominately inverse body regions.1 Moderate-to-severe HS is associated with various comorbidities, for example overweight and chronic obstructive pulmonary disease (COPD).2 Currently, adalimumab is the only biologic drug approved for HS by the European Medicines Agency.3 However, the drug survival time of adalimumab and other off-label biologics appears to be limited in HS.4 Therefore, alternative treatments are highly needed. We here present the first case of severe HS successfully treated with oral roflumilast, a selective phosphodiesterase (PDE)4 inhibitor.
In November 2020, a 54-year-old man was referred to our university dermatology clinic. Since 1995, he had experienced intermittent eruptions of boils and inflamed nodules in the intertriginous regions, and multiple systemic antibiotic courses of penicillin, dicloxacillin and doxycycline had been prescribed by his general practitioner. The patient had a 15-pack-year history of cigarette smoking and reported daily intake of a synthetic opioid (tramadol 50 mg, one to three times daily) due to skin pain. At clinical inspection, several inflamed interconnected tunnels, abscesses and excessive scarring were seen in inverse body regions, and the patient was diagnosed with Hurley stage 3 HS. In addition, moderate plaque psoriasis and obesity (body mass index 31·7 kg m−2) were observed.
Biologic therapy with adalimumab (80 mg subcutaneously every 2 weeks) was initiated, but as disease control was not achieved in 6 months, treatment was shifted to infliximab (5 mg kg−1 intravenously, every 4 weeks). Despite this, severe HS flares continued [International Hidradenitis Suppurativa Severity Score System (IHS4) = 16], and infliximab was discontinued after 5 months. Monotherapy with oral roflumilast 500 μg once daily was initiated (Figure 1a). At the 3-month follow-up, the patient reported markedly improved quality of life, less intake of tramadol due to reduced HS activity (IHS4 = 9) and complete clearance of his psoriasis. In addition, he had achieved a 9-kg weight loss (body mass index 29·1 kg m−2) (Figure 1b). Improvements were maintained after 5 months of treatment (IHS4 = 6) (Figure 1c). Apart from a brief episode of diarrhoea at treatment initiation, the patient experienced no side-effects, and therapy is currently ongoing.
Roflumilast is an orally administered small-molecule drug that inhibits the intracellular enzyme PDE4, and thereby alters expression of inflammatory mediators, including cytokines involved in HS pathogenesis.1, 5 Roflumilast was licensed first in class for severe COPD in 20115 and has recently shown potential in the treatment of psoriasis.6 Apremilast, another PDE4 inhibitor, was found to be efficacious and safe for HS in a randomized setting.7 Interestingly, the in-label list price of roflumilast is <7% that of apremilast in Denmark, with generic versions already available in some countries. Furthermore, experimental studies have found roflumilast to be up to 90 times more potent in inhibiting PDE4 isoforms compared with apremilast.8 In contrast to biologic therapy, oral roflumilast treatment does not require routine laboratory monitoring, and with proven efficacy in COPD and weight loss commonly reported during treatment, roflumilast may also work directly on associated HS comorbidities.5
In the present case, we observed considerable improvements in clinical HS presentation and quality-of-life measures with oral roflumilast therapy. In addition, the patient achieved a 10% weight loss, which may have contributed to the reduction of disease burden.1 Roflumilast could represent a novel and convenient treatment option for all severity stages of HS as well as associated comorbidities. To expand upon the rather limited treatment options in HS, larger studies investigating the long-term efficacy and safety of oral PDE4 inhibitors are warranted.
Author contributions
Simon Francis Thomsen: Conceptualization (equal); writing – original draft (equal). Mette Gyldenløve: Conceptualization (equal); writing – review and editing (equal). Claus Zachariae: Conceptualization (equal); writing – review and editing (equal). Hans Christian Ring: Conceptualization (equal); writing – original draft (lead); writing – review and editing (equal). Alexander Egeberg: Conceptualization (equal); writing – review and editing (equal).