Decline of programmed death-1-positive circulating T regulatory cells predicts more favourable clinical outcome of patients with melanoma under immune checkpoint blockade
Summary
Background
The role of T regulatory lymphocytes (Tregs) and their immunosuppressive mechanisms in the context of programmed death (PD)-1 blockade is not completely understood.
Objectives
To assess the impact of PD-1-blocking antibody treatment on Treg subpopulations in the blood.
Methods
We studied circulating Treg subpopulations in patients with melanoma under nivolumab or pembrolizumab treatment using flow cytometry and correlated these findings with clinical outcomes.
Results
These analyses revealed that the frequency of CD4+ CD25++ CD127− PD-1+ lymphocytes (PD-1+ Tregs) significantly decreased after the first cycle of immunotherapy (23% vs. 8·6%, P = 0·043). Compared with patients who did not show a significant decline of PD-1+ Tregs after the first treatment, those who did had better clinical outcomes with respect to progression-free survival (PFS, P = 0·022) and melanoma-specific death (MSD, P = 0·0038). Multivariate analysis confirmed that a significant decline of PD-1+ Tregs in peripheral blood after the first treatment cycle is a significant predictor of more favourable PFS and MSD (P = 0·04 and 0·017, respectively). Interestingly, the occurrence of immune-related adverse events was also an independent predictor for decreased risk of MSD (P = 0·047; odds ratio 0·064, 95% confidence interval 0·0042–0·97).
Conclusions
We provide preliminary evidence that circulating PD-1+ Tregs rapidly decline after the initiation of treatment with PD-1-blocking antibodies, which is associated with reduced risk of melanoma progression and MSD. Patients showing no decrease of these PD-1+ Tregs in peripheral blood are characterized by an impaired response to immune checkpoint blockade and worse outcome.
What's already known about this topic?
- Programmed death (PD)-1-blocking antibodies are highly effective in melanoma treatment.
- However, more than half of patients do not benefit from this therapy and to date it is difficult to predict which patients will respond to it.
What does this study add?
- PD-1-blocking antibody therapy rapidly results in a decline of circulating PD-1+ T regulatory cells (Tregs).
What is the translational message?
- Patients showing a decrease of PD-1+ Tregs appear to have better clinical outcome under PD-1 treatment.
Worldwide, melanoma is associated with more than 55 000 deaths per year. Once it has spread into the lymph nodes, visceral organs or brain, it becomes a life-threatening disease, despite the advent of molecular targeted therapy or immunotherapies.1 Particularly, immune checkpoint-modulating agents, such as the programmed death (PD)-1-blocking antibodies nivolumab and pembrolizumab, turned out to be highly effective in melanoma treatment.1 Hence, these innovative agents have been approved for the management of melanoma; however, more than half of patients do not benefit from this therapy, and to date it is difficult to predict which patients will not respond to it.
The PD-1 receptor is physiologically expressed by activated T cells, B cells, monocytes, natural killer cells and T regulatory lymphocytes (Tregs). It serves as an inhibitory receptor in cytotoxic cell activation after interaction with its ligands PD-L1 and PD-L2; its functional role in other cell subsets such as Tregs is less well established.2, 3 PD-L1 is expressed in several cells, including tumour cells and some host cells such as myeloid, lymphoid and epithelial cells.2, 3 The interplay between PD-1 and PD-L1 blocks CD8+ cytotoxic T-lymphocyte proliferation and survival, leads to apoptosis of tumour-infiltrating lymphocytes and promotes differentiation of CD4+ T lymphocytes into Tregs.1-3
Few studies have addressed circulating Tregs in different stages of melanoma. Correll et al.4 and McCarter et al.5 reported increased frequencies of Tregs in patients with advanced melanoma compared with healthy individuals. In a previous study we observed that the percentage of circulating CD4+ CD25++ CD127− T cells is an independent predictor for advanced melanoma, indicating a possible pathogenetic role of CD4+ CD25++ CD127− Tregs in melanoma progression.6 We aimed to study the impact of treatment with nivolumab or pembrolizumab on circulating Tregs and their subpopulations in patients with advanced melanoma, and to correlate the data with clinical outcome parameters.
Patients and methods
Patients
This prospective study was performed at the Skin Cancer Center of the Ruhr-University Bochum (Bochum, Germany) after having been approved by the local ethics review board of the Ruhr-University Bochum. We subsequently recruited patients with unresectable stage IIIc or IV melanoma who had an indication for anti-PD-1 treatment. Data were collected on patient characteristics (e.g. sex, age, tumour characteristics and initial imaging; Table 1) and pretreatment lab investigations including BRAF mutation analysis (cobas® 4800 BRAF V600 Mutation Test; Roche, Grenzach-Whylen, Germany). Blood parameters such as S100B and lactate dehydrogenase levels were routinely measured at the hospital, with cut-off values of 0·2 μg L−1 and 225 U L−1, respectively (Table 1). Complete work-up was regularly performed, including lymph node ultrasound, thoracic and abdominal computed tomography, and cranial magnetic resonance imaging. The criteria for treatment response were used in accordance with the immune-related response criteria.6 In order to rule out pseudoprogress, imaging was repeated after 6–8 weeks. Before and during therapy the patients were clinically monitored as recently recommended by Kähler et al.7
| Parameters | Data of 32 patients |
|---|---|
| Age (years), mean ± SD | 67·5 ± 13·7 |
| Sex: female; male | 11 (34); 21 (66) |
| Primary tumour thickness (mm), median (range) | 2·1 (0·14–8·9) |
| Melanoma stage | |
| Inoperable stage IIIc/d | 3 (9) |
| Stage IV | |
| M1a | 5 (16) |
| M1b | 10 (31) |
| M1c | 11 (34) |
| M1d | 3 (9) |
| S100B elevated: no; yesa | 19 (59); 13 (41) |
| LDH elevated: no; yesa | 17 (53); 15 (47) |
| BRAF mutation: no; yes; unknown | 24 (75); 5 (16); 3 (9) |
| Immunotherapy cycles, median (range) | 9·5 (1–56) |
| Immune-related adverse events | |
| None | 21 (66) |
| Grade 1 | 3 (9) |
| Grade 2 | 3 (9) |
| Grade 3 | 5 (16) |
| Grade 4 or 5 | 0 |
| Partial response | 8 (25) |
| Stable disease | 10 (32) |
| Disease control rate | 56% |
| Progression-free survival (months), median (range) | 4 (1–27) |
| Disease-specific survival (months), median (range) | 10 (1–36) |
| Disease-specific deaths | 12 (38) |
- Data are presented as n (%) unless stated otherwise. LDH, lactate dehydrogenase. aThe cut-off values for S100B and LDH are 0·2 μg L−1 and 225 U L−1, respectively.
Blood cells and flow cytometry for lymphocyte subsets
Blood collections, using ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, were performed 1 h before the initiation (baseline) of nivolumab or pembrolizumab and 1 h before each further therapy cycle 2- and 3-weekly, respectively. The FACSCanto IITM cytometer (BD Biosciences, San Jose, CA, U.S.A.), equipped with red (633 nm), blue (488 nm) and violet (405 nm) lasers, together with computer hardware and FACSDiva-softwareTM, were used to acquire and analyse lymphocyte populations. For daily performance check of the flow cytometer, CS&T beads (BD Biosciences) were applied. For Treg experiments, isotype control antibodies served as real negative controls. For correct instrument compensation, blood cells and/or BDcomp beads were used. In order to identify and determine the percentages of regular lymphocyte subtypes (T cells, B cells and natural killer cells) in the peripheral fresh blood, flow cytometric methods were predominantly used, as previously described in more detail,6 using antibodies for helper T lymphocytes (CD3+ CD4+), cytotoxic T lymphocytes (CD3+ CD8+), B cells (CD19+) and natural killer cells (CD3− CD16+ CD56+).
Direct immunofluorescent staining was done after lysing the red cells (BD FACSTM Lysing Solution). Absolute counts were captured using BD FACS Trucount tubes. For this regular phenotyping 5000 lymphocytes were counted. To analyse Tregs (CD4+ CD25++ CD127−) and the PD-1+ population within the Tregs, each time 20 000 T cells (CD3+) were recorded from fresh EDTA blood. The following fluorescence-conjugated antibodies were used: CD45 PerCP Cy5·5 (clone 2D1), CD3 phycoerythrin (SK7), CD4 fluorescein isothiocyanate (SK3), CD25 PE-Cy7 (ZA3), CD127 AlexaFluor647 (HIL-7R-M21) and CD279 (PD-1) BV421 (MIH4). Several fluorescence-minus-one controls were applied to discriminate negative and positive populations correctly. Leucocytes were first gated with a CD45/SS dot plot. Following on from CD3+ and CD4+ gating, the Treg population CD4+ CD25++ CD127− was used to look for PD-1-positive cells.
Statistics
Data analysis was performed using the statistical package MedCalc (Mariakerke, Belgium). Distribution of data was assessed by the D'Agostino–Pearson test. Non-normally distributed data were expressed as medians and range. Data were analysed using the Friedman anova, Mann–Whitney test, χ2-test and Spearman rank correlation procedure (univariate analyses). Parameters significant on univariate analysis were included in the multivariate analysis (logistic regression) in order to assess the relationship between lab parameters and outcome measures such as treatment response, melanoma-specific death (MSD) and progression-free survival (PFS). PFS and MSD were defined as the time from the first immunotherapy dose to the clinical event. P-values < 0·05 were considered significant.
Results
Immunotherapy outcome
From 32 patients, a total of 248 sequential blood draws were analysed (median number of draws per patient 6, range 2–29). The study population consisted of 11 women (34%) and 21 men (66%), and the mean ± SD age was 67·5 ± 13·7 years. Three patients (9%) had unresectable stage III melanoma and 29 of 32 (91%) had stage IV prior to starting treatment (Table 1). In five of 32 patients (16%) a BRAF mutation was found. Fourteen patients (44%) received nivolumab and 18 (56%) pembrolizumab. Two patients (6%) had BRAF inhibitor–mitogen-activated protein kinase kinase inhibitor combined therapy before immunotherapy, and one (3%) after immunotherapy. In eight of 32 (25%) a partial response was observed. Stable disease was seen in 10 of 32 patients (32%), resulting in a disease control rate of 56%. Disease progress was observed in 14 of 32 patients (44%) over time. The patients received a median 9·5 cycles (range 1–59) of immunotherapy. The median PFS was 4 months (range 1–27). A median survival of 10 months (range 1–36) was observed, corresponding to nine MSD events. Immune-related adverse events (irAEs, grade 1–3) were observed in 11 of 32 patients (34%). The occurrence of irAEs was significantly associated with decreased frequency of MSD (P = 0·018).
Pretreatment blood parameters
Baseline leucocytes, lymphocytes (absolute), lymphocytes (%), eosinophils, thrombocytes, neutrophil-to-lymphocyte ratio, thrombocyte-to-lymphocyte ratio, eosinophil-to-lymphocyte ratio and C-reactive protein were not significantly (P > 0·05) associated with response rates, PFS, MSD or adverse events. Elevated S100B levels prior to treatment start were significantly associated with poorer PFS (P = 0·0085) and MSD (P = 0·015). There was also a trend for such an association for elevated lactate dehydrogenase levels (P = 0·087).
Flow cytometry of lymphocyte subpopulations
As shown in Table 2, univariate analysis of lymphocyte subpopulations, including CD4+, CD8+ and CD4+ CD25++ CD127− Tregs, before and after the first cycle of therapy, revealed no significant differences (P > 0·05). This was also the case for comparisons over the course of treatment (12 cycles of either nivolumab or pembrolizumab), as assessed by Friedman anova (P > 0·05).
| Timepoint | CD3+ CD4+ | CD3+ CD8+ | CD4+ CD25++ CD127− | CD4+ CD25++ CD127− PD-1+ |
|---|---|---|---|---|
| Baseline | 45% (26–60) | 21% (9–46) | 9·4% (5·1–18) | 23% (3·4–61) |
| Favourable outcome: 26·9% (10–61·2) | ||||
| Unfavourable outcome: 8% (3·4–48·7) | ||||
| P = 0·075 | ||||
| After one cycle | 43% (26–60); P = 0·32 | 21% (9–48); P = 0·23 | 10·3% (4·5–20); P = 0·45 | 8·6% (0·1–61); P = 0·043 |
| Favourable outcome: 7% (0·1–23·8) | ||||
| Unfavourable outcome: 10·9% (6·4–61·4) | ||||
| P = 0·019 |
- The data are presented as the median (range) frequency.
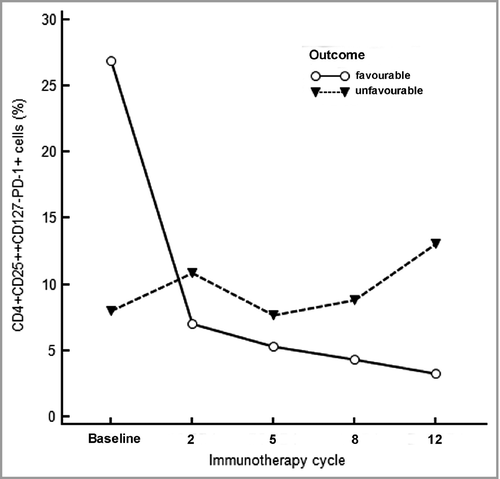
However, PD-1+ Tregs – median 23% (range 3·4–61) vs. 8·6% (range 0·1–61), P = 0·043 (Table 2) – significantly decreased after the first cycle of immunotherapy and maintained that decrease during the course of treatment. Figure 1 shows the flow cytometry graphs of two patients with different clinical disease outcomes. As also indicated in Figure 2, we observed a trend (P = 0·075) for higher baseline levels of PD-1+ Tregs in patients who experienced a decrease of PD-1+ Tregs after the first infusion compared with patients who did not. Patients who did show a significant decline of PD-1+ Tregs after the first treatment had a better clinical outcome with respect to PFS (P = 0·022) and MSD (P = 0·0038) than those who did not. Moreover, irAEs were associated with less occurrence of MSD (P = 0·018; univariate analyses).
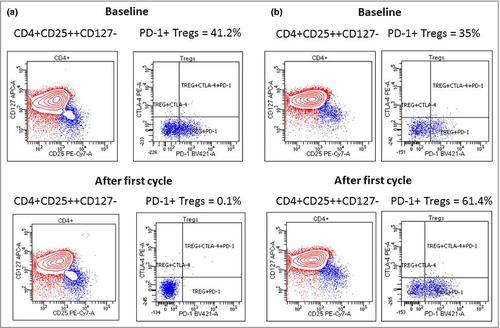
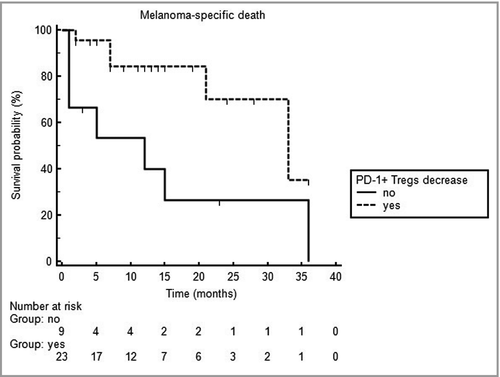
Multivariate analysis confirmed that a significant decline of PD-1+ Tregs in peripheral blood after the first treatment cycle is a significant predictor for more favourable PFS and MSD (P = 0·04 and 0·017, respectively), as indicated by odds ratios of 0·096 [95% confidence interval (CI) 0·010–0·9] and 0·058 (95% CI 0·0056–0·6), respectively. Interestingly, the occurrence of irAEs was also an independent predictor for decreased risk of MSD (P = 0·047; odds ratio 0·064, 95% CI 0·0042–0·97). As shown in Figure 3, decline of peripheral PD-1+ Tregs after the first treatment cycle turned out to be a significant (P = 0·013) independent predictor for reduced risk of MSD, as indicated by a hazard ratio of 0·27 (95% confidence interval 0·075–0·95).
Discussion
PD-1 is regarded as a negative regulatory signal in T lymphocytes. It has been demonstrated that Tregs play an important role in immune response regulation, becoming dysfunctional in autoimmunity and arguably hyperfunctional in the context of immune suppression in tumours. However, the role of Tregs and their immunosuppressive mechanisms in the context of PD-1 is not completely understood.8 Kumar et al.9 demonstrated that PD-1 expression on forkhead box (FOX)P3+ Tregs indicated greater suppressive capacity against CD8+ T-cell function in tumour, lung, spleen and draining lymph node than controls. Moreover, they showed that the interaction of FOXP3+ Tregs(high) PD-1(high) cells with PD-L1 induced immunosuppression by blocking CD8+ T-cell function in a prostatic tumour microenvironment.9 The authors concluded that FOXP3+ Tregs(high), PD-1(high) and CD8+(low) T cells may thus enhance tumour progression. Hence, targeting PD-1 on Tregs may be an effective approach to treat prostate cancer.9 Accordingly, Syed Khaja et al.10 suggested that breast cancer cells utilize Tregs, CD39, PD-1 and cytotoxic T lymphocytic antigen (CTLA)-4 molecules in creating an immune-subversive environment, suggesting a dual blockade of these immunosuppressive molecules as effective breast cancer treatment.10
Tregs exert their immunosuppressive effects through a variety of contact-dependent and contact-independent mechanisms, requiring different molecules and cytokines such as PD-1, CTLA-4, transforming growth factor-β and interleukin-10, among others.10 The study of Kim et al.11 recently demonstrated that PD-1-expressed tumour-infiltrating Tregs confers the suppressive function of proliferation in CD8+ T cells through PD-1–PD-L1 interaction. Shi et al.12 also reported that PD-1-expressing Tregs have selective and higher immunosuppressive ability than Tregs without PD-1 expression in mice. By contrast, Lowther et al.13 reported that high PD-1 expression on human Tregs identifies dysfunctional, exhausted Tregs secreting interferon-γ that exist in healthy individuals and are enriched in tumour infiltrates, possibly losing function as they attempt to modulate the antitumoral immune responses.13
The role of circulating PD-1+ Tregs in cancers is even less clear. van de Ven et al.14 showed in patients with lung cancer that the frequency of circulating PD-1+ Tregs was about 13%. Wang et al.15 did detect extensive (> 90%) intracellular PD-1 expression and 10–30% of surface PD-1 expression on isolated Tregs. The aforementioned frequencies of PD-1+ Tregs are similar to those observed in our study at baseline (median of 23%), which decreased rapidly after administration of PD-1-blocking antibodies.15, 16 Indeed, systemic administration of either nivolumab or pembrolizumab caused in the majority of patients a drastic decrease of circulating PD-1+ lymphocytes already after one treatment cycle, and this effect was widely maintained during the course of therapy in those patients benefiting from therapy. However, those patients who did not experience a decrease or even early increase of circulating PD-1+ cells had a poorer prognosis, as indicated by relatively high odds ratios for unfavourable PFS and MSD. Indeed, an early decrease of the PD-1+ Treg subpopulation after anti-PD-1 therapy appears to be an independent predictor for reduced risk of progression and MSD, as assessed by logistic regression.
However, another difference between the responder and the nonresponder groups is the baseline percentage of PD-1+ cells. Responders seem to have higher proportions of PD-1+ Tregs at baseline, which may be modulated by the PD-1 blockers, whereas nonresponder status is characterized by low or even increasing frequencies of circulating PD-1+ Tregs. Indeed, a biomarker before initiating therapy would be more valuable than an early biomarker after initiation of therapy. Although we observed a statistical trend (P = 0·075) for higher PD-1+ Treg frequency in patients with more favourable outcome, we could not detect a significant association between high PD-1+ Treg frequency at baseline and PFS or MSD. We can only speculate that the assessment of a larger study sample would have resulted in other findings, possibly identifying a high baseline frequency of PD-1+ Tregs as a significant predictor for more favourable outcome. Notably, we recently also studied the early effect of adjuvant nivolumab on circulating Treg subpopulations in patients with stage III melanoma.17 In line with the present study, circulating PD-1+ Tregs significantly decreased after the first cycle of immunotherapy and maintained that decrease during a 3-month course of treatment. By contrast, CTLA-4+ Tregs significantly increased after the first nivolumab dose compared with CTLA-4+ Tregs before the second treatment. Blood levels of PD-1+ Tregs and CTLA-4+ Tregs remained more or less decreased or increased during a 3-month course of therapy with nivolumab, respectively.17
It should also be noted that the objective response rate (25%) and the observed PFS (4 months) of the present patient cohort treated with PD-1-blocking antibodies in the real-life situation is inferior to the data observed in large randomized clinical trials, with response rates of about 40% and a PFS of approximately 5·5 months. This can be explained by different patient characteristics.1, 18, 19 However, another interesting finding of the present study is that patients who experienced irAEs had a better prognosis. In fact, the occurrence of irAEs as a potential prognostic biomarker for improved survival has recently been reported.20, 21 Nobashi et al.22 reported that irAEs detectable by [18F]-fluorodeoxyglucose positron emission tomography–computed tomography were useful for prediction of a favourable outcome. Early development of thyroiditis or vitiligo may particularly represent indicators of early response to immunotherapy.22 However, all in all it seems that correlation of irAEs with clinical efficacy is still controversial in metastatic melanoma, but it is more consistent in non-small cell lung cancer.23 Moreover, emerging data are supporting these findings in head and neck cancer. Hence, Saleh et al. concluded that further studies with larger numbers of patients and longer follow-up times are needed to validate the findings discussed above.23
In conclusion, we provide compelling evidence that circulating PD-1+ Tregs rapidly decline after the initiation of treatment with PD-1-blocking antibodies such as nivolumab and pembrolizumab. Most relevantly, patients who did show an early post-treatment decrease of PD-1+ Tregs were at reduced risk for disease progression and MSD. Hence, the determination of circulating PD-1+ Tregs potentially assists the prediction of treatment response to PD-1 blockers such as nivolumab and pembrolizumab.20 However, our preliminary data have to be confirmed in larger trials.
Acknowledgments
Open access funding enabled and organized by Projekt DEAL.




