Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis†
Plain language summary available online
Summary
Background
Genital psoriasis (GenPs) is a common, debilitating and difficult-to-treat manifestation of plaque psoriasis. However, few controlled, interventional studies of GenPs exist.
Objectives
To determine the efficacy of ixekizumab vs. placebo in patients with moderate-to-severe GenPs with ≥ 1% involved body surface area (BSA).
Methods
Patients with moderate-to-severe GenPs, defined as a baseline static Physician's Global Assessment of Genitalia (sPGA-G) score of ≥ 3, with BSA ≥ 1% were randomized 1 : 1 to receive placebo (n = 74) or the recommended dosing of ixekizumab (n = 75). Major outcomes included the percentage of patients achieving 0 or 1 scores on the sPGA-G (primary end point), overall sPGA, GenPs Sexual Frequency Questionnaire (GenPs-SFQ) item 2, and ≥ 3-point improvement from baseline on the GenPs itch numerical rating scale.
Results
At week 12, ixekizumab was superior to placebo for sPGA-G 0/1 (73% vs. 8%, P < 0·001), overall sPGA 0/1 (73% vs. 3%, P < 0·001), GenPs-SFQ item 2 score of 0 or 1 (78% vs. 21%, P < 0·001) and genital itch (60% vs. 8%, P < 0·001). No candidiasis was reported, no deaths occurred and one (1%) serious adverse event was reported in a patient receiving placebo.
Conclusions
Ixekizumab was superior to placebo for the treatment of moderate-to-severe GenPs with BSA ≥ 1%. The safety profile of ixekizumab was consistent with previous studies in moderate-to-severe plaque psoriasis.
Up to 63% of patients with psoriasis experience genital skin involvement at some point during the course of their disease.1 Genital psoriasis (GenPs) is a particularly challenging manifestation of psoriasis with significantly greater negative impacts on sexual health and quality of life than in patients with psoriasis with no genital involvement.1-3 Nevertheless, GenPs often remains undiagnosed due to insufficient evaluation of patients for genital involvement and a reluctance of patients and healthcare providers to discuss GenPs.4
The sensitive genital skin limits treatment options because many standard topical or ultraviolet-based treatments for psoriasis are either contraindicated or not well tolerated on genital skin. Current treatment guidelines support systemic therapies for GenPs when other treatment options fail, even in patients with lower body surface area (BSA) involvement.5 However, interventional clinical studies for GenPs are sparse.6-9 Therefore, there is an unmet clinical need for effective therapies for GenPs when topical therapies are ineffective or not well tolerated.
Ixekizumab results in rapid achievement of high clinical response rates that persist over long-term treatment of patients with moderate-to-severe plaque psoriasis.10-12 However, the efficacy of ixekizumab for GenPs is unknown. We report the efficacy and safety results from the 12-week blinded treatment period of a randomized, double-blinded, phase IIIb clinical trial investigating the impact of ixekizumab on GenPs severity, sexual health and health-related quality of life (HRQoL) in patients with ≥ 1% involved BSA and moderate-to-severe GenPs, as defined by a baseline score of 3 (moderate) or more on the static Physician's Global Assessment of Genitalia (sPGA-G).
The primary outcome measure (sPGA-G) was developed for assessment of the severity of GenPs and was validated using data from the screening and baseline visits of IXORA-Q. Herein we provide results for the assessment of the psychometric properties of the sPGA-G (Appendix S1, Tables S1 and S2; see Supporting Information). A description of the conceptualization, development methodology and content validity of the sPGA-G has been published previously.13
Patients and methods
Participants
Participants were ≥ 18 years old; had chronic plaque psoriasis for ≥ 6 months prior to baseline; had moderate-to-severe genital (sPGA-G ≥ 3) and overall psoriasis (overall sPGA ≥ 3); had plaque psoriasis in a nongenital area; had BSA involvement of ≥ 1% (up to 40% of enrolled patients could have BSA < 10%); were candidates for phototherapy or systemic therapy; had previously failed to respond to, or were intolerant of, at least one topical therapy for GenPs; and had agreed to use a reliable method of birth control.
Patients were excluded if they had forms of psoriasis other than plaque psoriasis, had pustules or vesicles in the genital area, or had previously received treatment with interleukin-17 antagonists. Additional key exclusion criteria included prior exposure to agents targeting α-4 integrin, live vaccination within 12 weeks prior to baseline, current or prior history of lymphoproliferative disease or malignant disease within 5 years prior to baseline, a recent history of suicide attempt (≤ 30 days) or a score of 3 on item 12 (thoughts of death or suicide) on the Quick Inventory of Depressive Symptomatology Self-Report (16 items) at screening or baseline or a history of histoplasmosis, coccidioidomycosis or any other infection typical of an immunocompromised host. Complete inclusion and exclusion criteria are available in Appendix S1 (see Supporting Information).
Study design
Participants were randomized 1 : 1 to ixekizumab (160 mg at week 0, then 80 mg every 2 weeks through week 12) or placebo during a 12-week blinded treatment period. Participants then entered a currently ongoing open-label treatment period through week 52.
IXORA-Q (Clinicaltrials.gov listing NCT02718898) was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent before undergoing study-related procedures.
Outcomes
Efficacy
The primary end point was to determine whether ixekizumab was superior to placebo at week 12 as determined by the percentage of patients achieving 0 or 1 (clear or minimal) on the sPGA-G.13 The major secondary end points were to determine whether ixekizumab was superior to placebo at week 12 as determined by the percentage of patients achieving 0 or 1 (clear or minimal) on the overall sPGA, a clinically meaningful (≥ 3-point) improvement from baseline for patients with ≥ 3 at baseline on the GenPs itch numerical rating scale (NRS),14 and a GenPs Sexual Frequency Questionnaire (GenPs-SFQ)15 item 2 score of 0 or 1 for patients with a score ≥ 2 at baseline. Additional secondary end points included mean change from baseline in the modified GenPs Area and Severity Index (mGPASI)1, 9 and in the Dermatology Life Quality Index (DLQI) at week 12. The percentage of patients achieving 0 or 1 on the DLQI at week 12 was also measured.
The GenPs-SFQ15 is a two-item, patient-reported outcome measure to assess the impact of GenPs on the frequency of sexual activity (including nonintercourse activities such as masturbation). Item 1 assesses the overall frequency of sexual activity by asking how often in the last week patients engaged in sexual activity (none/zero, once, or two or more times). Item 2 assesses the impact of GenPs on the frequency of sexual activity by asking how often during the last week GenPs symptoms limited the frequency of sexual activity; responses include never (0), rarely (1), sometimes (2), often (3) or always (4). Additional details on outcome measures are described in Appendix S1 (see Supporting Information).
Investigators were trained in the use of the sPGA-G for clinical assessment of GenPs through either an in-person or online training module, which was accompanied by a certification examination. Details on the training module and examination are described in Merola et al.13 The GenPs-SFQ item 2 and genital itch NRS were collected using an electronic diary that included instructions and guidance for self-assessment.
Safety
Safety was evaluated by monitoring of adverse events (AEs) for all enrolled patients. Treatment-emergent AEs (TEAEs) were defined as any unfavourable or unintended sign (including laboratory results), symptom or disease that first occurred or worsened on or after baseline. TEAEs were classified by severity, relationship with study drug or procedure, and association with patient discontinuation. AEs of special interest included cytopenias, liver function test changes or enzyme elevations, infections, injection-site reactions, allergic reactions or hypersensitivities, cerebrocardiovascular events, malignancies, inflammatory bowel disease and depression.
Statistical analysis
Sample-size determination
Study enrolment was planned for approximately 146 patients randomized 1 : 1 into treatment groups. As no biological drug had been evaluated in a large-scale, randomized, phase III clinical trial and no prior data existed for the sPGA-G, the sample size was calculated based on data from the assessment of DLQI item 9 (sexual difficulties due to skin) from phase II and phase III studies of ixekizumab in plaque psoriasis. Assumptions based on data from these studies included rates of 2% and 20% for skin-related sexual difficulties for the ixekizumab and placebo treatment groups, respectively. Power calculations used a two-sided Fisher's exact test at a 0·05 level of significance. With 146 patients (73 per treatment group) the study was calculated to have 94% power.
Efficacy and safety analysis
Efficacy analyses were performed for all randomized patients according to the treatment group to which they were assigned (intent-to-treat population). A gatekeeping strategy for testing the primary and major secondary end points was implemented to control the overall type I error rate at a two-sided alpha level of 0·05 (details are described in Appendix S1; see Supporting Information). There was no adjustment for multiple comparisons for any other secondary end points or time points.
Categorical efficacy and health outcome variables were analysed using a logistic regression model accounting for missing data using nonresponder imputation, in which patients were defined as nonresponders if they did not meet the clinical response criteria or had missing clinical response data for any reason including discontinuation at the analysis time point, unless otherwise specified. Continuous variables were analysed using a mixed-effects model for repeated measures (MMRM). Type III tests for the least square mean (LSM) were used for statistical comparisons between treatment groups for continuous variables. Both the logistic and MMRM models included baseline BSA category (1 to < 10% vs. ≥ 10%) as a covariate. Subgroup analysis for sPGA-G 0/1 response at week 12 using nonresponder imputation within each BSA subgroup (baseline BSA of 1 to < 10% or ≥ 10%) and sex (male or female) was performed.
Safety outcomes were summarized for all randomized patients who received at least one dose of investigational product according to the treatment group to which they were assigned. Safety data were summarized as the frequency of events in each treatment group.
Additional details of the statistical methods are described in Appendix S1.
Results
Patients
Patients (n = 149) were randomized from 5 May 2016 to 1 December 2016 to receive placebo (n = 74) or ixekizumab 80 mg every 2 weeks (n = 75) during the 12-week blinded treatment period. Overall, 99% (n = 74) of patients randomized to ixekizumab and 88% (n = 65) of those randomized to placebo completed the blinded treatment period (Fig. S1; see Supporting Information).
The baseline demographics and disease characteristics were similar between the placebo and ixekizumab treatment groups (Table 1). Overall, 75·8% of patients were male (the ixekizumab treatment group included one transgender patient in the male population who identified as female but had male genitalia). The mean ± SD age was 43·7 ± 12·7 years (range 19–77), the mean weight was 93·5 ± 24·7 kg and 39·6% of patients had 1% to < 10% BSA involvement. Previous systemic therapies were used by 52·3% (nonbiologics) and 19·5% (biologics) of patients. The inclusion criteria required all patients to have previously used topical therapies; 35·6% had previously used at least two topical therapies and 83·9% of patients had discontinued previous topical therapy for GenPs because of either inadequate response or loss of response.
| Placebo, n = 74 | Ixekizumab, n = 75 | |
|---|---|---|
| Age, years | 44·4 ± 12·6 | 43·1 ± 13·0 |
| Male, n (%) | 57 (77) | 56 (75)a |
| White, n (%) | 64 (86) | 67 (89) |
| Weight, kg | 95·1 ± 26·3 | 91·9 ± 23·1 |
| Previous systemic therapy, n (%) | ||
| Nonbiological systemic | 38 (51) | 40 (53) |
| Biological systemic | 15 (20) | 14 (19) |
| Phototherapy | 17 (23) | 20 (27) |
| Previous topical genital psoriasis therapy, n (%) | ||
| 1 therapy | 41 (55) | 55 (73) |
| 2 therapies | 22 (30) | 14 (19) |
| ≥ 3 therapies | 11 (15) | 6 (8) |
| Duration of genital psoriasis since onset, years | 8·3 ± 8·2 | 9·3 ± 10·0 |
| Duration of psoriasis since onset, years | 16·1 ± 12·5 | 16·9 ± 12·8 |
| sPGA-G scoreb | 3·5 ± 0·5 | 3·4 ± 0·6 |
| 3, n (%) | 41 (55) | 45 (61) |
| 4, n (%) | 32 (43) | 27 (36) |
| 5, n (%) | 1 (1) | 2 (3) |
| Overall sPGA score | 3·5 ± 0·6 | 3·5 ± 0·6 |
| 3, n (%) | 38 (51) | 41 (55) |
| 4, n (%) | 33 (45) | 30 (40) |
| 5, n (%) | 3 (4) | 4 (5) |
| mGPASI score | 28·3 ± 14·4 | 26·3 ± 15·4 |
| Genital itch numerical rating scale score | 6·0 ± 2·4 | 5·9 ± 2·4 |
| DLQI score | 13·4 ± 7·1 | 12·2 ± 7·2 |
| 0, n (%) | 0 | 0 |
| 1, n (%) | 0 | 1 (1) |
| GenPs-SFQ item 2 score | 2·0 ± 1·6 | 1·8 ± 1·7 |
| Involved body surface area, % | 16·8 ± 15·7 | 14·2 ± 12·6 |
| 1 to < 10%, n (%) | 28 (38) | 31 (41) |
| ≥ 10%, n (%) | 46 (62) | 44 (59) |
- Unless otherwise indicated, all values are presented as the mean ± SD. DLQI, Dermatology Life Quality Index; GenPs-SFQ, Genital Psoriasis Sexual Frequency Questionnaire; mGPASI, modified Genital Psoriasis Area and Severity Index; sPGA, static Physician's Global Assessment; sPGA-G, sPGA of Genitalia. aThe ixekizumab treatment group included one transgender patient in the male population who identified as female but had male genitalia. bOne patient in the ixekizumab treatment group was inadvertently enrolled with a baseline sPGA-G of 1. Therefore, this patient was not included in the count of patients with an sPGA-G score of 3, 4 or 5 in the ixekizumab group or the total study population.
Efficacy
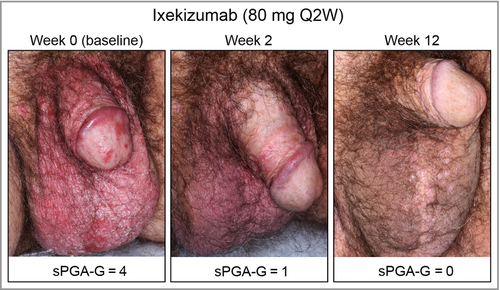
The primary and all major secondary end points were met. At week 12, significantly more patients achieved clear or almost clear genital skin (sPGA-G 0/1) with ixekizumab (73%) vs. placebo (8%, P < 0·001). Completely clear genital skin (sPGA-G 0) was achieved in significantly more patients receiving ixekizumab (56%) than placebo (5%, P < 0·001) (Fig 1a). Significant improvement was observed within 1 week of treatment with ixekizumab for both sPGA-G 0/1 (ixekizumab 24%, placebo 1%; P = 0·003) and sPGA-G 0 (ixekizumab 8%, placebo 0%, P = 0·028). At week 12, response rates for sPGA-G 0/1 were consistent across lower (1 to < 10%) and higher (≥ 10%) BSA subgroups with ixekizumab (BSA 1 to < 10%, 71%; BSA ≥ 10%, 75%), and were significantly greater than with placebo (both P < 0·001) (Fig. 1b). Similarly, week 12 sPGA-G 0/1 response rates were consistent between male and female patients, and response was significantly greater (P < 0·001) with ixekizumab (male 71%, female 79%) than with placebo (male 9%, female 6%). The LSM ± SE change from baseline in the mGPASI was significantly greater with ixekizumab at week 12 (ixekizumab −23·9 ± 1·48, placebo −3·9 ± 1·56; P < 0·001) (Fig. 1c). Figure 2 illustrates the clearance of GenPs for a patient randomized to ixekizumab.
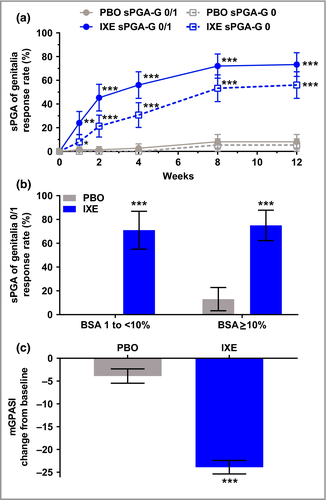
Ixekizumab resulted in significantly greater improvement in overall plaque psoriasis at week 12 vs. placebo for both overall sPGA 0/1 (ixekizumab 73%, placebo 3%; P < 0·001) and overall sPGA 0 (ixekizumab 40%, placebo 0%; P < 0·001) (Fig. 3a). Statistically significant improvements were observed with ixekizumab as early as weeks 1 and 4 for overall sPGA 0/1 (ixekizumab 17%, placebo 0%; P < 0·001) and overall sPGA 0 (ixekizumab 16%, placebo 0%; P < 0·001).
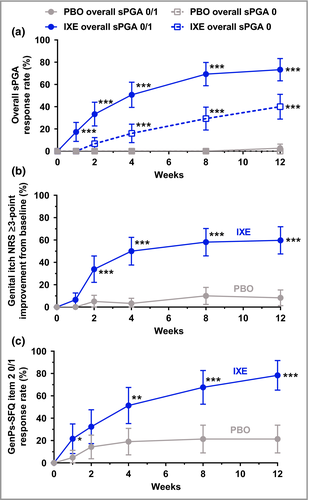
Significantly more patients achieved a clinically meaningful (≥ 3-point) improvement (reduction) from baseline in the genital itch NRS with ixekizumab (60%) vs. placebo (8%, P < 0·001) at week 12 among patients with a baseline score ≥ 3 (ixekizumab, n = 62; placebo, n = 60) (Fig. 3b). Statistically significant differences in genital itch were observed as early as week 2 (ixekizumab 34%, placebo 5%; P < 0·001). Definitions of response in the itch NRS are provided in Appendix S1 and Tables S3–S5 (see Supporting Information).
Among patients with a GenPs-SFQ item 2 score of ≥ 2 at baseline (ixekizumab, n = 37; placebo, n = 42), significantly more patients treated with ixekizumab achieved GenPs-SFQ item 2 score 0/1 (indicating GenPs never or rarely limited sexual activity) at week 12 (ixekizumab 78%, placebo 21%; P < 0·001) (Fig. 3c). Significant improvement with ixekizumab was observed as early as week 1 (ixekizumab 22%, placebo 5%; P = 0·036).
At week 12, HRQoL significantly improved with ixekizumab as determined by LSM change from baseline in DLQI (ixekizumab −9·7 ± 0·59, placebo −1·4 ± 0·62; P < 0·001) and DLQI 0/1 response rate (ixekizumab 45%, placebo 3%; P < 0·001) (Table 2). A score of 0 or 1 on the DLQI indicates no effect on HRQoL.
| Placebo, n = 74 | Ixekizumab, n = 75 | P-valuea | |
|---|---|---|---|
| DLQI total, LSM ± SE change from baseline | −1·4 ± 0·6 | −9·7 ± 0·6 | < 0·001 |
| DLQI score 0 or 1, n (%) | 2 (3) | 34 (45) | < 0·001 |
- DLQI, Dermatology Life Quality Index; LSM, least square mean. The mixed-effects model for repeated measures includes treatment, baseline body surface area category, baseline value, visit, treatment by visit and baseline value-by-visit interactions as fixed factors, with variance–covariance structure set to unstructured. aP-values are for the comparison of treatment effect between ixekizumab and placebo treatment groups at week 12, and are not adjusted for multiple comparisons.
Safety
Safety outcomes during the blinded treatment period are summarized in Table 3. TEAEs were more frequent with ixekizumab than with placebo, but were not significantly different between treatment groups (P = 0·19). Most TEAEs were mild or moderate in severity, with three (4%) severe TEAEs in the placebo group vs. one (1%) with ixekizumab. The most common TEAEs (those occurring at a frequency of ≥ 2% in the ixekizumab group and with greater frequency in patients receiving ixekizumab than placebo) were upper respiratory tract infection, injection-site reactions, oropharyngeal pain, pruritus, back pain, eczema and hypertension. Five (7%) patients receiving placebo and one (1%) receiving ixekizumab discontinued due to an AE. One serious AE (acute pancreatitis) occurred in a patient receiving placebo and resolved without discontinuation, and there were no deaths.
| Placebo, n = 74 | Ixekizumab, n = 75 | |
|---|---|---|
| TEAEs | 33 (45) | 42 (56) |
| Mild | 15 (20) | 23 (31) |
| Moderate | 15 (20) | 18 (24) |
| Severe | 3 (4) | 1 (1) |
| Serious adverse events | 1 (1) | 0 |
| Discontinuations due to adverse event | 5 (7) | 1 (1) |
| Death | 0 | 0 |
| Common TEAEsa | ||
| Upper respiratory tract infection | 5 (7) | 11 (15) |
| Injection-site reaction | 2 (3) | 8 (11) |
| Oropharyngeal pain | 2 (3) | 3 (4) |
| Pruritus | 2 (3) | 3 (4) |
| Arthralgia | 2 (3) | 2 (3) |
| Back pain | 0 | 2 (3) |
| Eczema | 0 | 2 (3) |
| Hypertension | 0 | 2 (3) |
| TEAEs of special interest | ||
| Hepatic | 2 (3) | 0 |
| Cytopenias | 0 | 0 |
| Infections | 13 (18) | 18 (24) |
| Allergic reactions/hypersensitivities | 3 (4) | 4 (5) |
| Injection-site reactions | 2 (3) | 8 (11) |
| Cerebrocardiovascular events | 0 | 0 |
| Malignancies | 0 | 0 |
| Depressions | 2 (3) | 0 |
| Inflammatory bowel disease | 0 | 0 |
- All values are presented as the number and frequency (%) of events. aCommon treatment-emergent adverse event (TEAEs) are defined as those that occurred more frequently in patients receiving ixekizumab than placebo and occurred at a frequency of ≥ 2% in the ixekizumab group.
Injection-site reactions were mild or moderate in severity and occurred more frequently in the ixekizumab treatment group (11%) than in the placebo group (3%). None of the injection-site reactions resulted in study discontinuation.
Treatment-emergent (TE) infections were more frequent with ixekizumab (24%) than with placebo (18%). Allergic or hypersensitivity reactions occurred at similar rates in the placebo (4%) and ixekizumab (5%) treatment groups. No cases of candidiasis or anaphylaxis were reported, there were no infection-related discontinuations and one patient discontinued due to allergic or hypersensitivity reactions. All TE infections and allergic or hypersensitivity reactions were mild or moderate in severity, except for one patient receiving ixekizumab who experienced two separate severe events during the blinded treatment period, reported by the investigator as a TE infection (infected eczema on the right thigh and right antecubital fossa) and an allergic or hypersensitivity reaction (exacerbation of eczema, resulting in study discontinuation). The sites of eczema were distant from the injection sites (abdomen) and this patient did not experience any injection-site reactions.
TE depression was reported in two patients (3%) in the placebo group, with no resultant discontinuation from the study. At baseline, 11 patients reported a prior history of suicidal ideation or behaviour (ixekizumab, three patients; placebo, eight patients), none of whom reported suicidal ideation or behaviour at any point during the blinded treatment period. Two patients (both receiving placebo) with no history of suicidal ideation or behaviour reported experiencing suicidal ideation or behaviour during the blinded treatment period.
Two patients (3%) in the placebo group developed hepatic-related TEAEs, one of whom discontinued due to an increased liver function test. There were no TE cytopenias, cerebrocardiovascular events, inflammatory bowel disease or malignancies reported during the blinded treatment period.
Discussion
Ixekizumab was superior to placebo for the treatment of moderate-to-severe GenPs during 12 weeks of treatment. Most (73%) patients receiving ixekizumab achieved complete or nearly complete resolution and 56% achieved complete resolution of GenPs. Consistently with previous clinical studies of ixekizumab, 73% of patients achieved overall sPGA 0/1, and 40% achieved overall sPGA 0 at week 12.10 Clinical response was rapid, with significant improvement as early as week 1 for sPGA-G 0/1, sPGA-G 0 and overall sPGA 0/1, and as early as week 4 for overall sPGA 0.
Ixekizumab resulted in clinically meaningful improvements in genital itch and HRQoL, and significantly reduced the negative impact of GenPs on the frequency of sexual activity at week 12. Nearly 60% of patients receiving ixekizumab (with a baseline genital itch NRS ≥ 3) displayed a clinically meaningful improvement at week 12, with significant improvement as early as week 2. Among patients who reported an impact on sexual frequency at baseline due to GenPs (GenPs-SFQ item 2 score ≥ 2), nearly 80% receiving ixekizumab improved to the point that their frequency of sexual activity was never or rarely limited by GenPs at week 12, with significant improvement observed as early as week 1. HRQoL also significantly improved with ixekizumab, with 45% of patients receiving ixekizumab reporting no clinically significant impact on HRQoL at week 12.
The overall safety profile of ixekizumab in this study was comparable with those reported in previous studies of ixekizumab in moderate-to-severe plaque psoriasis.10 Most TEAEs were mild or moderate in severity and discontinuations due to AEs were rare (one ixekizumab-treated patient). During the blinded treatment period, only one serious AE was reported, in a patient receiving placebo, and no deaths occurred.
The study population was globally diverse, with 149 patients enrolled from 34 sites in seven countries. This study is the only phase III, randomized, placebo-controlled, double-blinded clinical trial that has evaluated the efficacy of any therapy for the treatment of GenPs. Patients with both lower (1% to < 10%) and higher (≥ 10%) BSA involvement were included in this study. Thus, this study is among a small number of clinical trials investigating systemic therapy for plaque psoriasis in patients with < 10% BSA involvement where the severity and impact of the disease is considerable. A limitation of this study is that it did not include an active comparator, in part due to a lack of any well-established treatments for moderate-to-severe GenPs. Additionally, the study population was predominantly white and male. Lastly, this study had a relatively short duration. However, continued monitoring of the efficacy and safety of ixekizumab for GenPs is currently ongoing during an open-label extension period.
Although GenPs is a common and burdensome manifestation of plaque psoriasis,1-3 there are few published open-label studies that have evaluated the efficacy of interventional treatments for GenPs.7-9, 16 In this study, ixekizumab provided rapid and significant improvement in GenPs that was consistent across lower and higher BSA subgroups. Ixekizumab also provided rapid and significant improvement in the impact of GenPs on the frequency of sexual activity and in HRQoL.
Acknowledgments
We thank Clint Bertram, PhD, a medical writer and employee of Eli Lilly and Company, for writing support and Diane Richardson, an employee of Eli Lilly and Company, for her clinical operations management of the IXORA-Q study.
Appendix
Conflicts of interest statements.
C.R. has received honoraria for her role as a consultant or speaker for AbbVie, Boehringer Ingelheim, Dermira, Dr Reddy's Laboratories, Janssen, LEO Pharma, Lilly, Medimetriks, Novartis, Regeneron/Sanofi and UCB Pharma. A.M. has received grants and/or honoraria for his role as an advisory board member, consultant, investigator and/or speaker for AbbVie, Afecta, Allergan, Amgen, Anacor, Avillion, Boehringer Ingelheim, Celgene, Dermira, Eli Lilly and Company, Galderma, Janssen Biotech, LEO Pharma, Menlo, Novartis, OrthoDermatologics, Pfizer, Promius, Regeneron, Symbio/Maruho, Vitae and Xenoport. L.G. has received honoraria and grants as a speaker, consultant and investigator for Eli Lilly, LEO Pharma, Amgen, Celgene, Merck, Pfizer, AbbVie and Janssen; received honoraria as a speaker and consultant for Valeant and Tribute; and received grants as an investigator for UCB. A.B. has received honoraria and grants as an advisory board member and investigator for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly and Company, Genentech/Roche, GlaxoSmithKline, Janssen, LEO Pharma, Meiji, Merck Sharp and Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB, Valeant and Vidac; and honoraria as a paid speaker for Eli Lilly and Company, Janssen, Regeneron and Sanofi Genzyme. R. Bissonnette is an advisory board member and has received honoraria from AbbVie, Amgen, BMS, Janssen and Merck; is a consultant for and receives honoraria from AbbVie, Amgen, Celgene, Eli Lilly and Company, Galderma, Incyte, Janssen, LEO Pharma, Merck and Novartis; is a speaker for and receives honoraria from AbbVie, Amgen, Galderma, Janssen, LEO Pharma and Merck; and is an investigator for, and his institution receives grant support from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galderma, GSK Stiefel, Merck, Novartis, Pfizer, Kineta, Incyte, Janssen and LEO Pharma. K.M. is a consultant for and has received honoraria from Eli Lilly and Company and Eucerin and is an advisory member for Eucerin, the Netherlands. J.S. is a consultant and advisory board member for and receives honoraria from AbbVie, Celgene, Eli Lilly and Company, LEO Pharma and Novartis. J.C.C. receives grant support and honoraria as an investigator, speaker and advisory board member for AbbVie, Celgene, Eli Lilly and Company, Janssen, Regeneron-Sanofi Genzyme and Sun Pharmaceuticals; receives honoraria as a speaker and advisory board member for Actelion; and receives grant support as an investigator for Amgen and Boehringer Ingelheim. G.Y. receives honoraria as a consultant and scientific advisory board member for Eli Lilly, Opko, TREVI, Menlo, Sienna, Sanofi Regeneron, Novartis and Pfizer; and is supported by grants from LEO Foundation and Pfizer. A.B.G. receives honoraria as a consultant and advisory board member for Janssen, Celgene, Bristol-Myers Squibb, Beiersdorf, AbbVie, UCB, Novartis, Incyte, Eli Lilly and Company, Reddy Labs, Valeant, Dermira, Allergan and Sun Pharmaceuticals; and has received research or educational grants from Janssen, Incyte, Lilly, Novartis, Allergan and LEO Pharma. J.F.M. receives honoraria as a consultant for Biogen IDEC, AbbVie, Eli Lilly, Novartis, Pfizer, Janssen, UCB, Samumed, Science 37, Celgene, Sanofi Regeneron, Merck and GSK; is a speaker for AbbVie; receives grant support as an investigator for Biogen IDEC, Pfizer, Sanofi Regeneron, Incyte and Novartis; and has a licensed outcome measure with AbbVie and Eli Lilly. K.C.D. has received honoraria and/or grant support as a consultant and/or investigator for Amgen, AbbVie, Celgene, Pfizer, Novartis, Bristol-Myers Squibb, Eli Lilly, Xenoport, Sienna, Regeneron, Stiefel and AstraZeneca. S.F. has received grant support and honoraria as a paid investigator and speaker for Eli Lilly and Company, Janssen, Novartis, AbbVie and Celgene. O.O.O., R. Burge, A.N.N., F.E.Y., C.-Y.L., K.T. and A.P.B. own stock in and are employees of Eli Lilly and Company.




