The clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort
Summary
Background
BRAF and NRAS mutations are frequently found in melanoma tumours, and recently developed BRAF-targeted therapies demonstrate significant clinical benefit.
Objectives
We sought to investigate the clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort.
Methods
In total, 237 tumours, mostly metastatic lesions, from 203 patients were screened for mutations in exon 15 of BRAF and exon 2 of NRAS using Sanger sequencing. BRAF and NRAS mutation status was analysed in relation to clinical and histopathological characteristics, and outcome.
Results
Mutation in BRAF and NRAS was present in 43% (88% V600E, 10% V600K) and 30% (48% Q61K, 40% Q61R) of metastatic melanomas, respectively. We found consistent BRAF and NRAS mutation status in all but one of 27 patients with multiple metastases. BRAF mutation was associated with younger age at primary diagnosis (P = 0·02). Among patients with distant metastatic melanoma, patients with BRAF-mutant tumours without BRAF inhibitor treatment had inferior survival compared with patients with BRAF inhibitor treatment [hazard ratio (HR) 2·35, 95% confidence interval (CI) 1·10–5·01, P = 0·03]. We also observed a trend towards better prognosis for patients with wild-type and NRAS-mutant tumours compared with BRAF V600E-mutant tumours (HR 0·64, 95% CI 0·39–1·04, P = 0·07; and HR 0·76, 95% CI 0·48–1·21, P = 0·25, respectively).
Conclusions
We were able to confirm the effect of BRAF inhibitor treatment in a single clinical institution. The results suggest further that BRAF mutation is a weak prognostic factor but a strong predictive factor and that BRAF-mutant melanoma might constitute one or more distinct subtypes of the disease with certain aetiology and clinical outcome.
Constitutively activating BRAF mutations occur in approximately 40% of primary cutaneous melanomas.1 The most prevalent mutation is a T1799A transversion in exon 15 of the gene, which causes a V600E (Val600Glu) amino acid substitution in the protein.1 Mutation in BRAF leads to hyperactivity of the mitogen-activated protein kinase (MAPK)–extracellular signal-related kinase signalling pathway.2 Targeted therapy directed specifically towards BRAF V600E-mutated melanoma has demonstrated dramatic, although predominantly transient, clinical response.3 This highlights BRAF as an important clinical marker in the management of patients with melanoma. The second most commonly mutated oncogene in melanoma, NRAS, is mutated in 15–20% of cases and has been implicated as a prognostic factor in metastatic melanoma.4 Importantly, BRAF and NRAS mutations are mutually exclusive, and targeted therapeutic options for NRAS-mutant melanomas are under intense investigation.
Early studies have indicated that mutation in BRAF is an early event in melanoma development, and it has been hypothesized that BRAF mutation represents a separate pathway in the pathogenesis, reflecting a particular subtype of melanoma with a distinct phenotype and clinical outcome.5-10 In primary melanoma, BRAF mutation has been associated with younger age at diagnosis, localization to the trunk, absence of chronic sun damage, occult or one primary melanoma, and certain histopathological characteristics [presence of mitosis, superficial spreading melanoma (SSM) and low Breslow thickness],1, 7, 10 while NRAS mutation has been associated with thicker tumours and occurrence on the extremities.11 BRAF or NRAS status does not seem to predict overall survival or recurrence-free survival,10-14 but some studies point towards shorter survival for patients with BRAF-mutant metastatic disease.10, 13, 15, 16
In the current study, we aimed to determine the clinical significance of BRAF and NRAS mutations in a large cohort of surgically treated patients with metastatic melanoma. We sought to investigate the correlation to both clinical and histopathological characteristics, and outcome. Furthermore, we set out to determine the role of BRAF as a treatment-predictive factor.
Patients and methods
Patient selection and data collection
The study cohort was formed of a consecutive series of 237 patients with cutaneous malignant melanoma receiving surgical treatment between August 1993 and January 2012, where frozen tumour tissue had been collected. The vast majority had been surgically treated for metastatic disease at the Department of Surgery, Skåne University Hospital (SUS) in Lund. SUS is a referral hospital serving the Southern Healthcare Region of Sweden, with a population of 1·6 million people. Clinical and histopathological parameters were retrieved from patient clinical records, pathology reports and the National Population Registry. This study was approved by the local ethics committee of Lund University, Lund, Sweden (diary number 191/2007).
Twenty-two patients at our institution had received BRAF inhibitor treatment (vemurafenib, RO5185426; F. Hoffmann-La Roche Ltd, Basel, Switzerland) within clinical trials BRIM-3 (n = 1) or MO25515 (n = 21), via the Department of Oncology, SUS. Nine of them were found in our cohort and their frozen tissue samples were available for mutation screening. For the analysis of outcome from first distant metastasis, the patients treated with BRAF inhibitors formed a separate group. To increase the statistical power we here included the other 13 patients who had received the BRAF inhibitor, and for them testing was made on paraffin-embedded tissue.
BRAF and NRAS mutation testing
Genomic DNA was isolated from frozen melanoma tumour tissue using the AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany). Sequencing analysis was performed on polymerase chain reaction-amplified DNA by Sanger sequencing in both directions (forward and reverse) using previously described primers for exon 15 of BRAF, and codon 61 of exon 2 of NRAS.17 The sequencing traces were analysed using Sequencher v.4.5 (Gene Codes Corporation, Ann Arbor, MI, U.S.A.).
Statistical analysis
Survival curves were generated by the Kaplan–Meier method and P-values were calculated using the log-rank test. For correlations of characteristics to mutation status the Kruskall–Wallis test and χ2-test were used. To calculate hazard ratios (HRs), Cox univariate and multivariate analysis was used. P-values < 0·05 were considered significant and a 95% confidence interval (CI) was used in Cox regression analysis. The R software was used for all statistical analyses.18
Recurrence-free survival was defined as the time from primary melanoma to diagnosis of first metastasis. Distant-metastasis-free survival was defined as the time from first locoregional metastasis to diagnosis of first distant metastasis. Analysis was also made comparing overall survival from the date of diagnosis of the first distant metastasis to death/last follow-up.
Results
Overall, 34 patients were excluded from the cohort; six samples contained insufficient tumour material, four patients had insufficient follow-up data and 24 patients had incomplete BRAF and NRAS mutation test results. In total, 237 tumour samples from 203 patients were successfully screened for mutations in exon 15 of BRAF and codon 61 of exon 2 of NRAS. The patients had been referred to the Department of Surgery, SUS, for surgical removal of a melanoma tumour. Tumour tissue was obtained from 164 regional metastases, 13 local or satellite recurrences, 23 in-transit metastases, 25 distant metastases and 12 primary tumours (Table S1; see Supporting Information). Moreover, 13 patients (6%) had a history of multiple primary melanomas and 27 patients (13%) had an unknown primary tumour. More than one metastasis from the same patient was analysed for BRAF and NRAS status in 27 cases. In all cases but one, mutational status matched between the different metastases. The patient with a discordant mutation pattern had inguinal and iliac metastases removed at three different occasions within 31 months. The first two metastases were BRAF mutant and NRAS wild-type (WT), whereas the last metastasis was WT for both BRAF and NRAS after treatment with dacarbazine and sentinel-node-based adoptive immunotherapy. This patient was excluded from further analysis. In one case both the primary tumour and a lymph node metastasis were analysed from the same patient, and both contained an NRAS Q61K mutation and were WT for BRAF.
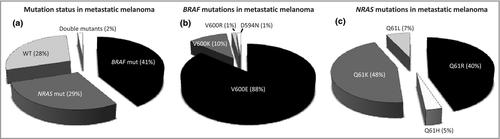
Of the 191 patients with metastatic melanoma lesions included in the analysis, 79 patients (41%) harboured mutations only in exon 15 of BRAF. The most common BRAF mutation was V600E (n = 71, 90% of BRAF-only mutations). Among the eight patients with non-V600E mutations, seven (9% of the total) harboured a V600K mutation and one V600R. In total, 55 of the 191 patients (29%) harboured mutations only in codon 61 of NRAS. Of these, 26 (47% of patients with NRAS mutations only) harboured the NRAS Q61K mutation, 23 (42%) Q61R, four Q61L and two Q61H. In addition, three patients harboured both BRAF and NRAS mutations (V600E/Q61K, V600K/Q61H and D594N/Q61K); in all other cases BRAF and NRAS mutations were mutually exclusive (Fig. 1). Among the 12 patients with primary melanoma tumours, four (33%) harboured BRAF mutation, all of which were V600E. Four of these patients (33%) harboured NRAS mutations, of which three were Q61R and one was Q61K. Furthermore, mutation frequency tended to differ depending on the type of recurrence. Distant metastases were most frequently BRAF mutated (64%), but not significantly so (P = 0·07, χ2-test), compared with regional metastases (42%), in-transit metastases (30%) and local recurrences (23%) (Table S1). There was no significant difference in NRAS mutation frequency depending on the type of recurrence, although in-transit metastases tended to be mutated most often (48%, P = 0·13, χ2-test).
The median time of follow-up from initial diagnosis was 6·5 years (range 0·02–23). The median time of follow-up after diagnosis of first recurrence was 3·7 years (range 0·04–19). At last follow-up 56 patients (28%) were alive with no evidence of disease, 22 patients (11%) were alive with metastasis and 124 patients (61%) had died.
Correlation to characteristics of primary tumour and clinical outcome
The association between patient and tumour characteristics and BRAF/NRAS mutations is shown in Table 1. The three samples containing both BRAF and NRAS mutations were excluded from further analysis. Patients with BRAF mutation were younger at primary melanoma diagnosis compared with patients with WT tumour and NRAS mutation (P = 0·02, Kruskall–Wallis test). BRAF mutation was more frequently found in metastases from SSM, while NRAS and WT tumours were associated with nodular melanoma, although not statistically significantly (P = 0·07, χ2-test). There was a nonsignificant trend (P = 0·12, χ2-test) for the trunk being the most common site of primary tumour in patients with BRAF-mutant melanoma. There was no significant correlation between BRAF/NRAS status and recurrence-free survival (P = 0·61, log-rank test).
| Characteristics | Wild-type (N = 58, 29%), n (%) | BRAF mutant (N = 83, 42%), n (%) | NRAS mutant (N = 58, 29%), n (%) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Female | 22 (38) | 31 (37) | 29 (50) | 0·27 |
| Male | 36 (62) | 52 (63) | 29 (50) | |
| Age at primary tumour (years), median (range) | 60 (28–89) | 56 (22–87) | 65 (32–80) | 0·02 |
| No. primary melanomas | ||||
| 1 | 45 (78) | 65 (78) | 49 (84) | 0·92a |
| > 1 | 5 (9) | 6 (7) | 2 (3) | |
| Occult | 8 (14) | 12 (14) | 7 (12) | |
| Site of primary melanoma | ||||
| Trunk | 21 (42) | 36 (51) | 17 (33) | 0·12b |
| Head and neck | 0 (0) | 1 (1) | 0 (0) | |
| Extremity | 28 (56) | 32 (45) | 33 (65) | |
| Not known | 1 (2) | 2 (3) | 1 (2) | |
| Breslow thickness, mm | ||||
| ≤ 1·00 | 4 (8) | 10 (14) | 5 (10) | 0·63 |
| 1·01–2·00 | 14 (28) | 18 (25) | 14 (27) | |
| 2·01–4·00 | 9 (18) | 18 (25) | 16 (31) | |
| > 4·00 | 19 (38) | 21 (30) | 13 (25) | |
| Not known | 4 (8) | 4 (6) | 3 (6) | |
| Histological subtype | ||||
| SSM | 10 (20) | 31 (44) | 11 (22) | 0·07c |
| NM | 19 (38) | 26 (37) | 23 (45) | |
| Other | 8 (16) | 3 (4) | 5 (10) | |
| Not known | 13 (26) | 11 (15) | 12 (24) | |
- a1 or > 1 vs. occult. bTrunk vs. extremity. cSuperficial spreading melanoma (SSM) vs. nodular melanoma (NM).
BRAF and NRAS mutations as prognostic and predictive factors in patients with metastatic melanoma
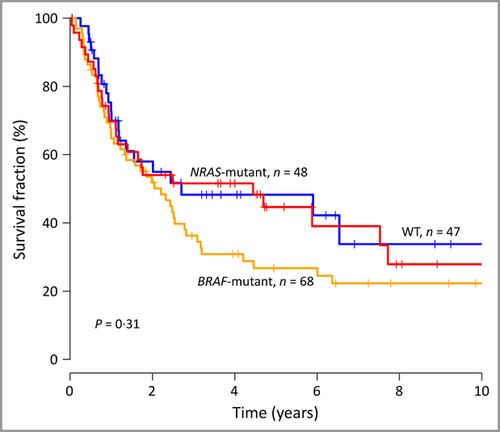
Patients with NRAS-mutant tumour were older at the time of first metastasis compared with patients with BRAF and WT tumours (P = 0·03, Kruskall–Wallis test, Table 2). There was no significant difference in the number of involved lymph nodes with respect to BRAF or NRAS status. Nevertheless, having more than one positive node was significantly associated with outcome (HR 1·78, 95% CI 1·13–2·80, P = 0·03). We investigated the clinical significance of BRAF/NRAS status from the first locoregional metastasis and found no significant difference in distant-metastasis-free survival or overall survival (P = 0·31 and P = 0·82, respectively, log-rank test; Fig. 2). Furthermore, no significant difference was found in clinical or histopathological characteristics, or in outcome, between Q61K and Q61R. The patient cohort was too small to make further analysis of BRAF/NRAS mutational subgroups.
| Characteristics | Wild-type (N = 58, 29%), n (%) | BRAF mutant (N = 83, 42%), n (%) | NRAS mutant (N = 58, 29%), n (%) | P-value |
|---|---|---|---|---|
| Age at first metastasis (years), median (range) | 62 (28–90) | 59 (21–88) | 66 (34–84) | 0·03 |
| Type of first metastasis | ||||
| Local/satellite | 3 (5) | 4 (5) | 3 (5) | 0·59 |
| In transit | 5 (9) | 7 (8) | 11 (19) | |
| Regional | 36 (62) | 55 (66) | 34 (59) | |
| Distant | 12 (21) | 15 (18) | 9 (16) | |
| None | 2 (3) | 2 (2) | 1 (2) | |
| No. involved lymph nodes | ||||
| 1 | 21 (58) | 26 (47) | 22 (65) | 0·29 |
| > 1 | 15 (42) | 28 (51) | 12 (35) | |
| Unknown | 0 (0) | 1 (2) | 0 (0) | |
| Age at first distant metastasis (years), median (range) | 62 (35–89) | 61 (21–88) | 67 (34–85) | 0·02 |
| Site(s) of distant metastasis involvement | ||||
| Lung | 14 (44) | 34 (55) | 17 (47) | |
| Liver | 16 (50) | 30 (48) | 14 (39) | |
| Bone | 7 (22) | 24 (39) | 12 (33) | |
| Central nervous system | 10 (31) | 19 (31) | 11 (31) | |
It has recently been suggested that BRAF mutation confers an inferior survival in stage IV melanoma compared with patients with the WT gene.10 Here, we wanted to investigate the significance of BRAF and NRAS in a clinic-based metastatic melanoma cohort. In total, 22 patients at our institution were enrolled in the BRIM-3 or MO25515 trials of vemurafenib and were included in the current study. Patients treated with the BRAF inhibitor tended to have a superior clinical outcome compared with patients with BRAF- or NRAS-mutant or WT tumours, who were not receiving the BRAF inhibitor (P = 0·13, log-rank test, Fig. 3a). When excluding patients with non-V600E-mutant tumour, the difference in outcome reached significance (P = 0·04 log-rank test, Fig. 3b). In a univariate Cox regression analysis, patients with BRAF-mutant tumours displayed a significantly poorer survival compared with patients treated with a BRAF inhibitor (HR 2·35, 95% CI 1·10–5·01, P = 0·03). There was a trend towards poorer survival for patients with NRAS-mutant and WT tumours compared with patients treated with a BRAF inhibitor (HR 2·07, 95% CI 0·95–4·54, P = 0·07 and HR 1·76, 95% CI 0·79–3·90, P = 0·16, respectively). When adjusting for age in a multivariate Cox regression analysis, the results were similar, and age did not appear as a significant prognostic factor. Furthermore, there was a weak trend for better prognosis in patients with WT and NRAS-mutant tumours compared with BRAF-mutant tumours (HR 0·74, CI 0·46–1·20, P = 0·22 and HR 0·88, CI 0·56–1·39, P = 0·58, respectively), which became more apparent after excluding non-V600E mutants (HR 0·64, 95% CI 0·39–1·04, P = 0·07 and HR 0·76, 95% CI 0·48–1·21, P = 0·25, respectively). Because all BRAF inhibitor treatment was given within clinical trials, thus representing a potential source of selection bias, we also made survival analysis after excluding patients diagnosed with stage IV disease after 31 December 2009, who might have been candidates for BRAF inhibitor treatment. The result was similar, with a nonsignificant difference in survival between patients with BRAF V600E- and NRAS-mutant, and WT tumours (P = 0·34, log-rank test).
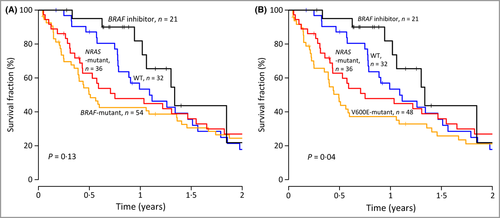
As the median survival in distant metastatic disease is < 1 year, we also investigated survival differences at 1 year. Here, a significant difference in survival between all four groups was identified (P = 0·007, log-rank test), and furthermore a nonsignificant trend to difference was found between BRAF-mutant and WT tumours (P = 0·07, log-rank test), while NRAS-mutant and WT tumours (P = 0·26, log-rank test) and BRAF and NRAS mutations (P = 0·46, log-rank test) demonstrated no difference in survival.
Discussion
This study describes BRAF and NRAS mutation status in a cohort of patients with metastatic melanoma treated at a single surgery department at a Swedish university hospital. The frequency of BRAF mutation in the cohort was in line with previous studies; 33% for primary melanoma and 43% for metastases.1, 4, 7, 10, 12, 16, 19, 20 The frequency of NRAS mutation was slightly higher compared with most other studies; 33% for primary melanoma and 30% for metastases. In our cohort the highest frequency of BRAF mutation was found in distant metastases; however, the difference was not significant, corroborating a previous study.19 Moreover, the authors of that study described consistency regarding BRAF/NRAS status in 76–91% of patients with multiple metastases.19 In the current study, we found consistent BRAF status in all but one of 27 patients with multiple metastases, and no discordant NRAS mutation. Although in a small cohort, such high concordance in BRAF status between paired metastases indicates a high treatment-predictive value of a positive BRAF test for systemic treatment with BRAF inhibitors. As expected, patients with tumours carrying BRAF mutation were younger and those with NRAS mutation were older at diagnosis of primary melanoma and first metastasis compared with patients with WT tumours. BRAF-mutant melanomas tended to be associated with SSM, while NRAS mutations more often presented in nodular melanoma, in line with previous findings.1, 4, 7, 10, 12, 16 BRAF mutation has also been associated with trunk location1, 7, 10 and to some extent the lower extremities,7 and we found a similar trend in this respect. Our cohort included very few head/neck melanomas, as those are treated at the ear, nose and throat clinic at SUS. As head/neck melanoma has been associated with V600K mutation,12 this might explain the low percentage of V600K mutation in our cohort, and also partly the high frequency of NRAS mutations. Several recent studies have indicated that V600K mutation, which is the second most common BRAF mutation after V600E, might represent up to 20–30% of BRAF-mutant melanoma tumours.4, 12, 21
In total, we found 72% of melanoma metastases to harbour mutation in either BRAF or NRAS, leaving 28% of metastases to be WT. Only three tumours were mutated for both genes, and one of these tumours harboured a BRAF D594N mutation. Indeed, a recent study indicated mutual mutations in BRAF and NRAS in tumours harbouring non-V600E BRAF and non-Q61 NRAS mutations.22 Many studies have aimed to identify the genetic alterations in melanoma tumours WT for BRAF and NRAS. In a recent study using whole-exome sequencing of 121 melanoma samples, NF1 was recurrently mutated in WT samples.22 Furthermore, alterations in the KIT oncogene are found in rare melanoma subtypes (such as mucosal and acral). Interestingly, both NF1 and KIT are functionally connected to the MAPK pathway, suggesting that activation of this pathway is a key event in the development of melanoma. Further studies are needed in order to determine the association between clinical features and NF1 mutation.
To investigate further the prognostic information of BRAF and NRAS status, we analysed the clinical outcome from the time of distant metastasis to death. In this analysis patients receiving a BRAF inhibitor formed a separate group, as clinical trials have found BRAF inhibitors to have a clinical benefit for patients with melanoma.3, 23, 24 We could demonstrate a significantly better survival for patients treated with BRAF inhibitors compared with other patients with BRAF mutation. There was also a trend for better prognosis for patients treated with BRAF inhibitors compared with others with NRAS mutation. As far as we know, we are the first to describe the survival of patients treated with BRAF inhibitors and untreated patients in relation to both BRAF and NRAS status. Among patients not treated with BRAF inhibitors, a trend for shorter survival in patients with BRAF V600E compared with WT and NRAS melanoma was observed, although it was not statistically significant. Others have described a similar trend comparing patients with BRAF mutation and the WT gene.10 By excluding the few tumours with non-V600E mutation, the group of patients with BRAF-mutant tumours was made more homogeneous and more representative for analysis. Although the difference in survival became more apparent after this exclusion, the excluded tumours are too few and heterogenic to draw any conclusions of the prognostic significance of a non-V600E mutation. However, this implies that BRAF V600E is a distinct subtype of melanoma affecting prognosis. In all, these results suggest that BRAF mutation is a weak prognostic factor but a strong predictive factor in metastatic melanoma. Further molecular research in tumour cohorts, as we described here, will aid in identifying novel prognostic and treatment-predictive factors.
As the cohort consists of patients who were considered to benefit from surgical treatment, there might be a selection bias towards patients with less aggressive or more localized disease, which might skew the results. However, the patients and tumour characteristics are well in line with other studies based on patient registers.
In conclusion, this study confirms previously described associations between BRAF and NRAS mutation status and age. Importantly, we found a high concordance of BRAF and NRAS mutation status between paired metastases diagnosed at different time points. We were able to confirm the effect of BRAF inhibitor treatment in a single clinical institution. Moreover, we describe a trend for worse survival for patients with BRAF V600E-mutated tumours compared with BRAF WT and with NRAS mutations. Hence, these results suggest that BRAF mutation is a weak prognostic factor but a strong predictive factor and that BRAF-mutant melanoma might constitute one or more distinct subtypes of the disease with certain aetiology and clinical outcome. It also highlights NRAS as an important target in the search for new molecular therapy.




