Righting ability in hatchling turtles does not predict survival during dispersal in the field
Abstract
While many studies use laboratory-based whole-organism performance measures as proxies for fitness, the implicit assumption that better laboratory performance translates into higher fitness in the wild is rarely tested. Righting response in turtles is frequently quantified in the laboratory and interpreted as either a measure of coordination or a direct proxy for survival. Here, we quantify two aspects of the righting response (latency time and active righting time) of hatchling red-eared sliders (Trachemys scripta elegans) in the laboratory and perform a release experiment to measure survival at a critical, terrestrial life-history stage. We found no relationship with active righting time or latency with the number of days hatchlings took to reach the drift fence. We also found no directional selection during dispersal from nest to water on either active righting time (the most commonly reported righting metric) or latency to right. We detected disruptive selection acting on latency time, although latency time also was shown to be highly sensitive to experimental perturbations. Our results, however, do not shed light on the relationship of these performance measures with survival at other life stages, and further studies clarifying the relationship between righting and survival in the field are warranted.
Introduction
Quantifying whole-organism performance under controlled settings is a common and useful practice for ecologists, physiologists, and evolutionary biologists. Whole-organism performance is defined as the ability of an animal to conduct an ecologically relevant task, and these performance traits typically integrate morphological, physiological, and potentially behavioural aspects of the organism (Irschick et al., 2008). Performance traits have been measured for a wide variety of purposes, including documenting developmental plasticity (Elphick & Shine, 1998; Kaplan & Phillips, 2006), estimating thermal performance curves (Huey & Stevenson, 1979; Logan, Cox & Calsbeek, 2014), and comparing athletic potential in human athletes (Pienaar, Spamer & Steyn, 1998; Wilson et al., 2016).
Rigorous statistical methods for measuring selection in the wild originally related morphology to fitness (Lande & Arnold, 1983), and were extended to highlight that morphology influences fitness through performance (Arnold, 1983). While quantifying performance traits has been useful for many applications, biologists often operate under the untested assumption that higher laboratory performance translates into higher fitness in nature (Irschick & Garland, 2001). Yet, research assessing whether performance in the laboratory co-varies with fitness has shown that this translation is not always the case. In some instances, selection on performance traits has been documented, while others have found weak or no selection (reviewed in Irschick et al., 2008).
The righting response in turtles is a performance trait that is frequently quantified as a proxy for fitness (Fisher, Godfrey & Owens, 2014; Sim, Booth & Limpus, 2014; Steen et al., 2015). This performance measure entails placing a turtle upside down on its carapace and measuring how long it takes to right itself (Davy, Paterson & Leifso, 2014). The righting response can be quantified as the entire time period a turtle is inverted, or can be partitioned into the time actively trying to right and time spent motionless before attempting to right. The righting response is used because it is easy to quantify, turtles are motivated to perform, and there is substantial variation in performance (Freedberg et al., 2004). Righting ability is frequently interpreted as a trait that directly or indirectly influences fitness. In some cases, it is considered a trait that is directly relevant to turtle survival (Burger, 1976; Finkler & Claussen, 1997; Steyermark & Spotila, 2001; Golubovic et al., 2013). In other words, turtles that are slow to right themselves are more vulnerable to predation, desiccation, or other threats. There is evidence that sea turtles become inverted regularly during dispersal, and that these inversions slow their dispersal (Steyermark & Spotila, 2001). Others interpret righting as a general index of physical fitness (i.e. an indirect correlate of fitness), as it measures strength, coordination or neuromuscular development that may be relevant to many aspects of its life (Burger, 1998; Colbert, Spencer & Janzen, 2010). Despite the considerable number of studies that have quantified righting performance in the laboratory, only one other recent study has investigated whether an aspect of this trait influences survival (Carter et al., 2016) and none, to our knowledge, has determined the strength or form of selection acting on the trait in the field (Delmas et al., 2007; Refsnider, 2013).
In this study, we quantify two aspects of the righting response (latency time, active righting time) of hatchling red-eared sliders (Trachemys scripta elegans) in the laboratory. Subsequently, we assessed recapture fates at a drift fence as a proxy for survival during dispersal from nesting areas to nearby aquatic habitats. The dispersal stage is characterized by high predation and mortality, and previous studies at our field site have documented consistent selection on morphology and performance during this stage (Janzen, Tucker & Paukstis, 2000a, 2007). This study allowed us to examine whether metrics of righting performance in the laboratory are under selection during a crucial life-history stage in the field. Specifically, we predicted that turtles that attempted to right themselves sooner and successfully righted themselves faster would have a potential survival advantage.
Methods
We studied a population of red-eared sliders (Trachemys scripta elegans) in west-central Illinois, USA. Females in this population typically lay several clutches of eggs in mid-May through early July, mainly within agricultural fields and natural field borders. Eggs hatch in late summer, yet hatchlings remain in the nest overwinter. The following spring (typically late April and May), hatchlings emerge from the nests and disperse to nearby aquatic habitats.
Over 38 days during the nesting season in 2008, we hand-captured 111 gravid females during their nesting migration near Grafton, Illinois and followed the oviposition and incubation procedures similar to Tucker (2000) and Janzen et al. (2000a). Briefly, we brought females to a nearby field station where egg laying was induced by injection of oxytocin (Ewert & Legler, 1978). After oviposition, we released females at their capture location. We incubated eggs in nine plastic sweater boxes containing a 1:1 ratio of vermiculite and water by mass. Sweater boxes were stored in an uninsulated room near the study site and were rotated daily to minimize thermal gradients. Incubation temperatures fluctuated with local ambient air temperatures to simulate natural nest conditions. Due to the range of oviposition dates, some variation in thermal conditions experienced by eggs occurred (i.e. earlier laid clutches were exposed to different conditions than later laid clutches).
Upon hatching, we transported turtles to Iowa State University (ISU). Hatchlings were marked using a Sharpie marker pen on the carapace with clutch and individual number for identification in the laboratory; however, these temporary markings were not sufficient for the release experiment. Hatchling turtles have unique plastron patterns, and plastrons were scanned to individually identify hatchlings (Myers, Tucker & Chandler, 2007). Scans were printed and these printouts were visually inspected to identify turtles captured at the drift fence if the Sharpie markings were illegible.
We distributed siblings randomly among different plastic dishes for overwintering to minimize clutch effects. Dishes were moistened with deionized water every 2 weeks. Hatchlings were stored at room temperature until late November, when we gradually reduced incubator temperatures to a constant 7 °C, where they remained throughout hibernation. We gradually raised incubator temperature over 21 days to 20 °C prior to testing righting performance. We performed righting trials between 8 and 15 April 2009.
To measure righting performance, we used 11 opaque, round plastic dishes (11.7 cm diameter × 6.4 cm depth) in an array below a video camera so we could test multiple turtles simultaneously. We quickly placed each turtle on its carapace, and subsequently exited the room. All trials lasted 20 min, and turtles that did not right themselves in that time (N = 53) were excluded from further analyses (as in Freedberg et al., 2004), although their recapture fates are summarized in the results. Trials were performed in a temperature-controlled room at 20.4 ± 0.4 °C, and turtles were housed in the same room as the trial, so temperature acclimation was not an issue. Although many other studies measure righting performance at higher temperatures, this temperature is well within the range typically experienced during dispersal at the field site (weather station data from the National Weather Service ID ALNI2, Cooperative observer ID 110137).
We primarily quantified two aspects of the righting response for each turtle: latency time and active righting time. Latency time is the time elapsed from when the turtle was placed on its carapace until the time it began to attempt to right itself. Active righting time is the amount of time the turtle was actively attempting to right itself. This was the total amount of time from when the turtle first attempted to right itself until it succeeded, minus any time the turtle was motionless within that period (Freedberg et al., 2004). Some researchers view the entire time period from when the turtle was inverted to when it successfully rights as an important righting metric. Accordingly, we also measured this third aspect of righting time, which we call total time. Total time is the time elapsed from when the turtle was placed on its carapace until it completed righting (as measured in Carter et al., 2016). This total time value would differ from the sum of the latency and active time values only if a turtle was not successful in righting itself in the first attempt, and subsequently laid motionless on its carapace before attempting to right again. Because multiple turtles were tested simultaneously, we quantified measures separately for each individual by watching the same video multiple times, focusing on a different turtle each time.
We inadvertently introduced an inconsistency in the specific method of placing the turtle on carapace. Some turtles were placed in the dish right side up first, and then moved onto their carapace to begin the trial. Other turtles were directly placed on their carapace, such that the trial began immediately as they were placed in the dish. Accordingly, we accounted for this methodological discrepancy in our statistical analyses.
Additionally, a camcorder malfunction resulted in a subset of hatchling righting trials never being recorded. Therefore, we did not obtain behavioural data for all individuals available for the 111 clutches. Despite this issue, 970 individuals from 111 clutches contained morphological, behavioural, and survivorship data. Additionally, a subset of turtles (N = 60) was righted twice to quantify repeatability with at least 1 h between measurements.
After righting trials were completed, we performed a field-based release experiment to assess a proxy for the likelihood of survival. Turtles were transported from Iowa State to the field site in Illinois to examine their probability of recapture during field dispersal from the nesting area to aquatic habitats. All hatchlings were weighed just prior to release. We initiated the release on 29 April 2009 at 08:00 under ideal conditions for hatchling migration [light rain, with partial sun and temperatures in the low 20s (°C) range]. We released turtles in a large group 40 m out from the centre of a 285 m drift fence that was aligned North to South, paralleled a large, water-filled ditch connected to further aquatic habitat, and contained 20 pitfall traps identical to that described in Tucker (2000) and illustrated in Tucker (1997). The fence was ~50 m from the ditch (thus the hatchlings were released 90 m from the ditch). We righted turtles if they overturned while we released them and organized them into a single line, but did not orient them in a specific direction (Janzen, Tucker & Paukstis, 2000b). Turtles were released after a rain event, and some ‘natural’ turtles were captured in pitfall traps as well. These ‘natural’ turtles were not used in our experiment, but their presence in the pitfall traps indicated our release trial coincided with the natural migration of hatchlings from nest to water. We checked pitfall traps twice a day for recaptured hatchlings, and continued checking traps 5 days beyond when the final wild hatchling was captured, which was 8 days after any of our experimental hatchlings were recaptured (last hatchling captured 7 May 2009). We identified recaptured hatchlings by comparing the unique patterns on their plastrons to the printouts described above. Hatchling turtles are very effective at orienting towards water (Warner & Mitchell, 2013), and our drift fence was sufficiently long that hatchlings likely did not go around the fence (Janzen et al., 2000b). Although it is likely that some turtles did not orient correctly, this is unlikely to qualitatively influence our results. Incorrect orientation likely results in death because there is no suitable habitat for aquatic hatchling turtles in any direction except for towards the drift fence. Moreover, removing predators from this site more than doubles recapture rates, suggesting mortality from predation is the primary cause of non-recapture rather than poor orientation (Tucker, Paukstis & Janzen, 2008) Therefore, hatchlings that were not recaptured live were presumed dead. Live hatchlings that were caught in the drift fence were released the day following capture into the Illinois River (similar to Tucker, 2000).
Statistical analyses
To examine whether survivors and non-survivors differed in body mass measured prior to release, we performed a t-test. Body mass and carapace length were largely collinear (ρ = 0.86, P < 0.001, Spearman rank correlation), thus, we use mass as a proxy for hatchling size. We recorded three aspects of righting performance from the videos: latency (time until first movement), active righting time (time spent attempting to right), and total time (all time from inverted until righted). Latency time and total time were highly collinear (ρ = 0.95, P < 0.001, Spearman rank correlation). Additionally, 71% of hatchlings only attempted righting themselves once during the trial, and in such instances total time is exactly the sum of active time and latency time. Thus, the total time variable was redundant with the other righting metrics in the model, and thus was excluded from the final models selected.
Wilcoxon rank sum test was used to assess differences in latency time and active righting time between survivors and non-survivors. To determine whether placement method substantially contributed to variation in active righting time and latency, we performed separate analyses of variance using clutch, overwintering cup, and placement method as categorical variables. We also performed Kruskal–Wallis rank sum tests using only placement method as the independent variable and active righting time or latency as the dependent variables. We used Spearman rank correlations to determine whether oviposition date influenced incubation duration or righting performance, and Kruskal–Wallis tests to determine if incubation box influenced righting performance.
We performed both directional and nonlinear selection analyses on body mass, latency, and active righting time. Latency time and active righting time were not normally distributed, and transformations did not improve normality; however, logistic regression is robust to deviations in normality (Janzen & Stern, 1998). Body mass, latency time, and active righting time were standardized to a mean of zero and unit variance, and used as predictor variables in a logistic regression, with survival as the response variable (Janzen & Stern, 1998). Since placement method appeared to have a weak effect on latency (see Results) we included placement method as a categorical variable to account for the variation attributable to this inconsistency in our methods. We visualized selection surfaces with cubic splines (Schluter, 1988). We also assessed nonlinear selection, such as that experienced in stabilizing selection, by performing a similar analysis, but including the squares of standardized mass, latency time, and active righting time as additional variables. We multiplied these nonlinear selection gradients by two (Stinchcombe et al., 2008).
For the surviving turtles, we recorded how many days it took from release to reach the drift fence. We used Spearman rank correlation to assess whether body mass, active righting time, or latency time was related to the time to reach the fence. To assess whether active righting time or latency time was related to the time to reach the fence when including body mass and placement method in the model, we also used a general linear model (GLM) with a Quasi-Poisson distribution to account for overdispersion.
Additionally, a subset of individuals (N = 60) was tested for righting performance twice (with each trial having identical placement methods). To test rank repeatability of righting performance, we used Spearman rank correlations (Davy et al., 2014). In addition, we calculated repeatability from intraclass correlation coefficients (Lessells & Boag, 1987) using the sum of squares from an ANOVA with active righting time or latency time as the response variable and individual ID as the independent variable. For the individuals with multiple measures, we chose the first measure for inclusion in the selection analyses, as this measure is more comparable with the measurements for all other hatchlings that were only tested a single time.
Results
Our primary dataset consisted of 970 hatchlings, of which 469 were recaptured alive in the drift fence, nine were discovered dead, and 492 were not recaptured and presumed dead (48% survival). The release was initiated in the morning of 29 April 2009 and the last hatchling was recaptured on 7 May 2009. Ninety-seven per cent of the survivors were found at the drift fence within 3 days of the release, with most individuals found at the drift fence on the day of release (N = 330) or the first or third day after release (N = 53 and 73, respectively). Between one and four individuals were found at the drift fence per day on day 2 and days 4–8 of the release. Beyond the eighth day after the release, no other experimental hatchlings were found. Of the 53 additional individuals did not successfully right themselves in the righting assay (and were removed from other analyses), 27 survived and 26 were not recaptured (51% survival). Thus the survival of the hatchlings that righted themselves in the trials and those that did not are very similar.
Survivors were heavier than non-survivors, and there were no differences in active righting time or latency time between survivors and non-survivors (Table 1). Latency time and active righting time were weakly associated (ρ = 0.055, P = 0.087) via Spearman rank correlations. Mass and active righting time were weakly, significantly correlated (ρ = −0.092, P = 0.004,). In other words, heavier hatchlings spent less time actively righting. Mass was not correlated with latency (ρ = −0.029, P = 0.369). Overall, we do not anticipate that colinearity between variables will cause issues in our phenotypic selection analyses (sensu Mitchell-Olds & Shaw, 1987).
| Trait | Survivors | Non-survivors | Test statistic | P-value |
|---|---|---|---|---|
| Mass (g) |
5.60 g (5.52; 1.15) [2.62, 8.64 g] |
5.41 g (5.52; 1.15) [1.83, 8.21 g] |
t(968) = −2.53 | 0.011 |
| Righting time (s) |
3.42 s (2; 4.32) [1, 44 s] |
3.74 s (2; 5.88) [1, 89 s] |
z = −1.2163 | 0.223 |
| Latency time (s) |
273.55 s (196; 291.48) [0, 1724 s] |
290.82 (214; 268.76) [0, 1612 s] |
z = −1.9176 | 0.055 |
- A t-test was used for mass, and a Wilcoxon rank sum test was used for latency and righting time. In parentheses is the median and standard deviation separated by a semicolon. Ranges for each variable are given in brackets.
Using an ANOVA with clutch, cup, and placement method as independent variables, neither active righting time nor latency was significantly influenced by placement method (righting: F = 0.85, d.f. = 1, P = 0.236; latency: F = 2.90, d.f. = 1, P = 0.090). Clutch significantly influenced both traits (righting: F = 1.68, d.f. = 110, P = 0.010; latency: F = 1.69, d.f. = 110, P > 0.001), while overwintering cup significantly influenced latency time (F = 3.32, d.f. = 95, P > 0.001) but not righting time (F = 0.85, d.f. = 95, P = 0.913).
Turtles placed directly on their carapace exhibited qualitatively longer latency and active righting times (latency directly placed: mean = 397.4, median = 298, SD = 330.3; flipped: mean = 206.2, median = 153, SD = 208.6; active righting time directly placed: mean = 4.1, median = 2, SD = 5.8; flipped: mean = 3.2, median = 2, SD = 4.7). Latency and active righting time are both strongly non-normal. Thus, we also performed Kruskal–Wallis rank sum tests, which indicated a relationship between active righting time and placement method (χ2 = 4.43, d.f. = 1, P = 0.035) and latency and placement method (χ2 = 123.22, d.f. = 1, P < 0.001). Latency was potentially more affected by differences in methodology because the time from when the turtle was placed on its back and when the researcher left the room was extended when turtles were placed directly on their backs into the cups. Thus, we felt that placement method may be an important factor to take into account in phenotypic selection analyses.
Spearman rank correlations revealed no relationship between the time to reach the fence and body mass (ρ = −0.053, P = 0.251), latency time (ρ = 0.008, P = 0.871), or active righting time (ρ = 0.230, P = 0.619). When using a GLM with a Quasi-Poisson distribution and testing significance against a chi-squared distribution, days to reach the fence was not related to latency (deviance = 4.042, d.f. = 1, 466, P = 0.193) or active righting time (deviance = 1.636, d.f. = 1, 465, P = 0.408), but did exhibit a statistically significant relationship with body mass (deviance = 9.473, d.f. = 1, 467, P = 0.046).
While we did not monitor temperatures inside the nine incubation boxes, we found that clutches with Julian oviposition dates that were later during the season exhibited shorter incubation times, suggesting that later clutches experienced warmer incubation temperatures than earlier clutches (Spearman rank correlation, ρ = −0.795, P < 0.001). The date of oviposition was not correlated with active righting time or latency (active righting time: ρ = −0.016, P = 0.619; latency: ρ = 0.039, P = 0.221). Incubation box did not have any influence on righting time or latency (Kruskal–Wallis χ2 = 11.5685, d.f. = 8, P = 0.1715; Kruskal–Wallis χ2 = 6.3501, d.f. = 8, P = 0.6081, respectively), but these boxes were filled in a random way and included clutches from across the season in a single box.
Our multivariate logistic regression analysis did not detect significant directional selection on either active righting time (βavggrad = −0.02, P = 0.493) or latency time (βavggrad = −0.03, P = 0.328), although body mass was under significant positive directional selection (βavggrad = 0.08, P = 0.015) (Fig. 1). Nonlinear selection was not detected for mass (γ = −0.01, P = 0.850) or active righting time (γ = −0.02, P = 0.903) although latency time showed a pattern of selection consistent with disruptive selection (γ = 0.27, P = 0.011). Placement method was not significant in either selection analysis (P > 0.4, in both cases).
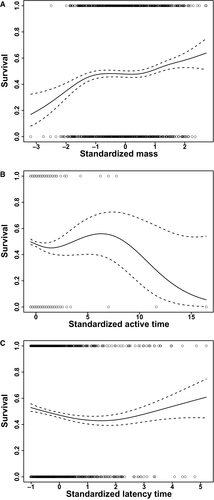
Latency time was significantly rank repeatable (ρ = 0.449, P < 0.001), although active righting time was not (ρ = 0.160, P = 0.221). Latency time and active righting time both had significant repeatability when analyzed via the method detailed in Lessells & Boag (1987) (r = 0.415, P < 0.001 and r = 0.246, P = 0.027, respectively).
Discussion
Researchers frequently quantify whole-organism performance in the laboratory for various purposes, including as a proxy for fitness. The righting response in turtles is one such trait, yet whether performance in righting is under selection in the field remained unexamined until our study. In this study, we quantified two aspects of righting performance of hatchling red-eared slider turtles (Trachemys scripta elegans), and found that active righting time in the laboratory (the primary metric of interest in most studies) was not related to survival (recapture probability) during dispersal from terrestrial nesting habitat to a drift fence that served as a proxy for aquatic habitat. Latency time showed a pattern consistent with disruptive selection. However, our laboratory assay on latency was sensitive to various methodological factors in our experiment. Thus, we interpret this disruptive selection finding with caution.
The nesting habitat to water dispersal stage is characterized by high predation on hatchling turtles, and much research has focused on natural selection acting on body size during this stage (Janzen, 1993; Mitchell, Warner & Janzen, 2013). Research at our site has consistently supported the ‘bigger is better’ hypothesis, showing that natural selection favors larger individuals because they disperse faster and have reduced exposure to terrestrial predators than smaller individuals (Janzen et al., 2000a, 2007; Myers et al., 2007). Because ecological performance (dispersal speed) is directly related to survival and natural selection is fairly consistent across space and time during this stage (Janzen et al., 2000b), our system provided an excellent opportunity to test whether laboratory performance is an appropriate fitness proxy.
If righting performance is important for survival during this stage, we predicted that turtles that attempted to right themselves sooner and did so faster would have a survival advantage. The prediction with active righting time is straightforward; more coordinated animals should survive better if this trait is important during dispersal. The prediction with latency time is less straightforward. Although remaining immobile may be advantageous at times for cryptic animals (Janzen, 1995), we expect bright yellow plastron of our species, only visible when the animal is inverted, would increase detection probability of inverted hatchlings. Consequently, we predict shorter latency times would be advantageous. However, our analyses revealed no significant directional selection on either trait, and there was no relationship between righting performance and ecological performance of survivors (i.e. how quickly the survivors reached the drift fence).
Active righting time is often invoked as a correlate of physical strength and coordination, and of fitness, yet active righting time was not under selection in our release. Prior laboratory work investigated whether latency and active righting time were influenced by a number of experimental factors, and provided both interesting and variable results (Delmas et al., 2007). For example, they observed that survival during a year in the laboratory was related to active righting time when eggs were incubated under fluctuating conditions. Such a laboratory relationship would suggest righting performance is a suitable indirect measure of fitness (as no predators were present to attack inverted, vulnerable individuals). The authors also suggested the righting metric was useful to identify particularly low quality individuals, but could not distinguish between medium and high quality individuals. Field tests, such as ours, would be more appropriate to distinguish between higher quality individuals. Similarly, active righting performance, as a proxy for coordination, could be correlated with other locomotor performances that may be linked with fitness. Booth, Feeney & Shibata (2013), however, found that self-righting performance is not correlated with swimming performance in sea turtle hatchlings, likely because the head and neck are of primary importance in mechanically righting the turtle, but are not important for swimming. Similarly, we found no relationship between righting performance in the laboratory and field performance.
Our results are consistent with the one other study we are aware of that relates righting performance to field survival (Carter et al., 2016), although methodological differences exist. They evaluated whether certain chemical perturbations during incubation (e.g. exogenous corticosterone application) influenced aspects of development, and their experiment included righting trials and a field release. They used total time (rather than latency and/or active righting time), and total time was not related to survival. Our general results accord with the results from their study, and suggest righting performance may not by predictive of survival during hatchling dispersal from nest to aquatic habitat.
Our analyses investigating variation in latency time identified some unexpected results. Nonbiological factors within our study, such as placement method and overwintering cup, induced some variation in latency time. This observation illustrates the sensitivity of latency time to these methodological factors in our experiment, and other researchers have noted the sensitivity of this trait as well (Delmas et al., 2007). There is substantial methodological variation in measuring the righting response across other experiments (i.e. whether or not an observer is present in the room during the righting trial; Freedberg et al., 2004; Davy et al., 2014) which could also influence latency times. Thus, in general, we suggest researchers take caution when measuring latency time.
Due to the underlying concerns with the sensitivity of latency time to various factors in our experiment, we proceed with reservations while interpreting the results of the selection analysis. We did not detect directional selection on latency time, but did observe significant nonlinear selection consistent with disruptive selection. This is an unexpected pattern, as it suggests turtles with very short, or very long latency times have a slight survival advantage. While it has been demonstrated previously in running trials that immobility in turtles favored first year survivorship in a hatchling snapping turtles, hatchling snapping turtles are highly cryptic (Janzen, 1995). Slider turtles have bright yellow plastrons, which are exposed when they are inverted and may reduce their visual extremely long latency had a survival advantage over the intermediate turtles, as such a behaviour would both increase their overall exposure and decrease their crypsis, at least when latency to right is interpreted as a direct measure of performance. If taken as an indirect measure of propensity to move, turtles with long latency times may be advantageously immobile and ‘hide’ when threatened by predators. Indeed hatchling sliders are known to bury themselves and wait for an opportune time to complete dispersal (Tucker, 2000). However, due to our aforementioned concerns with the latency data, we suggest that further research examining the consistency of this pattern is warranted before broad conclusions are drawn with respect to latency.
Our analysis of rank repeatability on a subset of individuals demonstrated that while latency was rank repeatable, active righting time was not. A recent study that was designed to evaluate the rank repeatability of righting time also demonstrated that total righting time varies substantially among trials, and is generally not rank repeatable (Davy et al., 2014), which further suggests that total righting may not be a good measure of fitness. However, using standard measures of repeatability (Lessells & Boag, 1987) both latency and active righting time were significantly repeatable in our study.
The results from multivariate logistic regression indicate that natural selection favored heavier individuals. Our observation of significant positive selection on body mass was consistent with prior research at our field site (Janzen et al., 2000b). Of the survivors, our GLM analysis indicated a relationship between mass and field performance (time to reach the fence), although the Spearman rank correlation did not reveal such an effect. This relationship, which shows larger hatchlings reach the fence faster, has previously been well demonstrated (Janzen et al., 2000b). Given there was significant selection on body size, we believe this population during the time period of our study is under the same selection pressures observed in other studies. While the positive selection on body size is interesting, it has been well documented and thoroughly discussed elsewhere (Janzen, 1993; Janzen et al., 2000a, b, 2007).
It is well appreciated that incubation temperature can alter performance and morphology of hatchling reptiles (Riley, Freedberg & Litzgus, 2014; Mitchell, Maciel & Janzen, 2015). Our study design allowed realistic temperature fluctuations to occur daily and throughout the season, adding biological relevance that is often lost from constant incubation temperatures (Warner & Shine, 2011; but see Paitz et al., 2010). We incubated eggs in a common location in nine sweater boxes, which reduced the range of thermal conditions eggs would have encountered naturally. However, oviposition date produced meaningful variation in incubation conditions. Clutches with later oviposition dates had shorter incubation durations, suggesting they experienced warmer incubation conditions. The conditions of our experiment were ecologically relevant, and the active righting times observed in our study are similar to those of a closely related species (C. picta) incubated in natural nests (Riley et al., 2014). Still, had we exposed eggs to a broader range of incubation conditions, we could have induced more variation in righting response (Freedberg et al., 2004). This variation could have amplified our ability to detect a relationship between righting response and survival, and is an important caveat to our study and its interpretation.
The utility of laboratory performance measures usually depends on the measure's ability to predict fitness in nature (Husak et al., 2006; Irschick et al., 2008). Our study found that active righting time is not predictive of survival during hatchling dispersal of an aquatic turtle. Righting performance may be directly associated with fitness for turtles in other life stages (e.g. nesting forays, mating). It is more likely that righting performance is highly relevant for terrestrial species. For example, several species of tortoises have antagonistic interactions between males that result in frequent inversions (Mann, O'Riain & Hofmeyr, 2006), and righting performance is likely directly relevant to survival in these species. In fact, shell geometry of many high-domed terrestrial species has been shaped by selection to a nearly optimal shape for righting in adults (Domokos & Várkonyi, 2008).
Red-eared sliders often disperse greater distances (~500 m; Tucker, 2000) than other aquatic turtles and hide as an anti-predator behaviour. Sliders that complete this dispersal more quickly reduce their exposure to predators and have higher survival. Thus, walking speed, endurance, or other anti-predator behaviours may be more relevant than righting performance during the nest to water migration for this species. One potential caveat to our work is that our drift fence was located at a considerably shorter distance (40 m) from the release site than slider turtles typically travel from nest to water (~500 m; Tucker, 2000); thus, selection on righting time or latency may be revealed over larger hatchling dispersal distances. In fact, our drift fence was positioned 50 m inland from the river, so even the recaptured turtles in our experiment could have perished over this longer distance. Further research on the utility of righting performance in other species and other ecological situations will shed light on the generality of our findings.
Here we challenged the common assumption that laboratory performance correlates with survival in the wild. Our results indicate that active righting time is not under selection during dispersal of aquatic hatchling turtles, and we suggest it may be a poor proxy for hatchling fitness during the dispersal from nest to water. However, the numbers of studies exploring the relationship between righting performance in the laboratory and performance and/or fitness in the field are few, and each study has its limitations. Thus, ample opportunity to expand upon this area exists. We encourage further research that either quantifies performance in the field (Irschick & Garland, 2001; Irschick et al., 2008) or examines the relationship between laboratory performance and survival in the field. Such research will provide a valuable platform for interpreting laboratory performance measures in the future.
Acknowledgements
We thank L. Flewelling for help with the righting trials and F. Janzen for support and access to materials. We thank three anonymous reviewers for comments that enhanced this article. Work was performed under ISU IACUC=9-08-6630-J. Turtles were collected by John Tucker while employed by the Illinois Department of Natural Resources. TSM was partially supported by the NSF (DBI-1402202).




