Ecogeographical patterns of morphological variation in pygmy shrews Sorex minutus (Soricomorpha: Soricinae) within a phylogeographical and continental-and-island framework
Abstract
Ecogeographical patterns of morphological variation were studied in the Eurasian pygmy shrew Sorex minutus aiming to understand the species’ morphological diversity in a continental and island setting, and within the context of previous detailed phylogeographical studies. In total, 568 mandibles and 377 skulls of S. minutus from continental and island populations from Europe and Atlantic islands were examined using a geometric morphometrics approach, and the general relationships of mandible and skull size and shape with geographical and environmental variables were studied. Samples were then pooled into predefined geographical groups to evaluate the morphological differences among them using analyses of variance, aiming to contrast the morphological and genetic relationships based on morphological and genetic distances and ancestral state reconstructions, as well as assess the correlations of morphological, genetic, and geographical distances with Mantel tests. We found significant relationships of mandible size with geographical and environmental variables, fitting the converse Bergmann's rule; however, for skull size, this was less evident. Continental groups of S. minutus could not readily be differentiated from each other by shape. Most island groups of S. minutus were easily discriminated from the continental groups by being larger, indicative of an island effect. Moreover, morphological and genetic distances differed substantially and, again, island groups were distinctive morphologically. Morphological and geographical distances were significantly correlated, although this was not the case for morphological and genetic distances, indicating that morphological variation does not reflect genetic subdivision in S. minutus. Our analyses showed that environmental variables and insularity had important effects on the morphological differentiation of S. minutus.
Introduction
Ecogeographical ‘rules’ describe general trends in morphology and related traits along geographical gradients. Recently, there has been a renewed interest in developing a more comprehensive and integrative understanding of the generality of these trends and the mechanisms that cause them (Lomolino et al., 2006; McNab, 2010).
Two of the best-known ecogeographical rules are Bergmann's rule and the island rule. In its original form, Bergmann's rule states that warm-blooded vertebrate species (or races or populations within a species) from cooler climates tend to be larger than congeners from warmer climates (Bergmann, 1847; Blackburn, Gaston & Loder, 1999). This vaguely defined rule, later reformulated to refer to populations within species or to species in a monophyletic higher taxon, describes a positive relationship between body size and latitude (Mayr, 1963; Blackburn et al., 1999; Meiri, 2011). The island rule predicts an increase of body size for small mammals (gigantism) and a decrease of body size for large mammals (dwarfism) in island populations compared to mainland populations (Van Valen, 1973). Although it has been argued that Bergmann's rule is a valid generalization (Ashton, Tracy & Queiroz, 2000; Meiri & Dayan, 2003), there are species data showing the opposite trend (the converse Bergmann's rule) and a lack of support (nonsignificant results) from a large number of species (Ashton et al., 2000; Meiri & Dayan, 2003). Similarly, the validity of the island rule has been questioned because most studies have used poor size indices, as well as very large islands or mainland populations only distantly related to the island populations (Lomolino, 2005; Meiri, Dayan & Simberloff, 2006; Meiri, Cooper & Purvis, 2008), and there is also a large number of studies that report evidence against it (Raia & Meiri, 2006; Meiri et al., 2008; Meiri, Raia & Phillimore, 2011). Furthermore, McNab (2010) argued that geographical patterns in size variation should not be subdivided into different ecological rules but rather be considered as aspects of the same phenomenon concerning the differential allocation of energy and physiological responses to resource availability.
Considering the controversy associated with these ecogeographical patterns, more comprehensive intra- and interspecific studies are needed to determine their validity and basis (Lawlor, 1982; Lomolino, 2005; Gaston et al., 2008; Meiri et al., 2008). This includes paying careful attention to anomalous findings because they may reflect distinctive features that highlight causal explanations, or the use of combined approaches important for developing an integrative understanding of biogeographical patterns and the generation of hypotheses (Lomolino et al., 2006).
In the present study, we use the Eurasian pygmy shrew Sorex minutus (Linnaeus, 1766; Soricomorpha: Soricinae) as a model species for investigating different ecogeographical patterns along geographical, climatic, and environmental gradients in continental and insular settings, and in a phylogeographical context. Sorex minutus has a broad geographical distribution in continental Eurasia, from Lake Baikal in Siberia to Southern, Central, and Northern Europe, and in the British Isles (Mitchell-Jones et al., 1999). It is found in very different habitats, such as alpine and northern tundra, forests, shrub lands, swamps, heaths, and grasslands (Hutterer, 1990). The phylogeographical history has been investigated in detail. Six mitochondrial (mt)DNA lineages with discrete geographical distributions have been described (Mascheretti et al., 2003; McDevitt et al., 2010, 2011; Vega et al., 2010a, b), with support from Y-chromosome markers (McDevitt et al., 2010, 2011): four Southern European lineages distributed within the three European Mediterranean peninsulas, namely the ‘Iberian’, ‘Italian’, ‘South Italian’, and ‘Balkan’; a ‘Northern’ clade distributed from Lake Baikal to Central and Northern Europe, and also found in Britain; and a ‘Western’ clade found in the Pyrenees, Northern Spain (Cantabria Mountain Range), Western France, Ireland, and in the periphery of Britain and islands off the western and northern coast of Britain, forming a ‘Celtic fringe’ (Searle et al., 2009; McDevitt et al., 2011). The Northern and Western lineages colonized Britain sometime after the Last Glacial Maximum over the land bridge with continental Europe (Vega et al., 2010a; McDevitt et al., 2011) and the Western lineage colonized Ireland within the last 10 000 years via a human-mediated introduction (McDevitt et al., 2009, 2011).
We explored the two questions. (1) What is the morphological diversity of S. minutus throughout its European range; in particular, are there geographical, climatic, and/or environmental patterns in continental Europe and/or relating to island occupancy in the British Isles? (2) To what extent does the morphological diversity in continental Europe and the British Isles resemble the phylogeographical pattern detected with molecular markers? Accordingly, we used a geometric morphometric approach (Rohlf & Marcus, 1993) combined with environmental and phylogeographical information to investigate the biogeography of S. minutus, one of the many small mammals that are widespread in Europe but for which remarkably little effort has been made to document or understand their non-molecular geographical variation using modern methodologies.
Geometric morphometrics is a method for the study of form (the shape and size of an object) based on Cartesian landmark coordinates, where the geometry of the configuration of landmarks is preserved throughout the analysis (Zelditch et al., 2004; Mitteroecker & Gunz, 2009). Combined with genetic, ecological, environmental, and taxonomical information, geometric morphometrics is an exceptionally powerful tool for studying intraspecific variation (Loy, 1996; Zelditch et al., 2004; Nogueira, Peracchi & Monteiro, 2009; Vega et al., 2010b) and has great potential for our understanding of ecogeographical patterns.
Material and Methods
Collection and digitization of samples
We acquired S. minutus specimens from our own fieldwork ethically collected (Sikes, Gannon & the Animal Care and Use Committee of the American Society of Mammalogists, 2011), from owl pellets and from museum and private collections (see Supporting information, Appendix S1, Table S1). In total, we analyzed 568 mandibles and 377 skulls from continental and island sites in Europe (Fig. 1). Photographic images of the external side of left hemi-mandibles and the left half of the ventral side of skulls were taken using a digital camera at a fixed distance. Mandibles were placed flat under the camera lens. Skull samples were placed on a purpose-built polystyrene and Plasticine cradle, leaving the ventral side parallel to the lens, judged by eye. A small piece of graph paper was included as a scale in each photograph and the sample was placed in the middle of the image area to avoid parallax.
Morphological analyses on the mandible and skull data sets were carried out using TPS-SERIES software (by F. J. Rohlf; available at: http://life.bio.sunysb.edu/morph). Eighteen landmarks were placed on the external side of left hemi-mandibles and 19 landmarks were placed on the left half of the ventral side of skulls using TPSDIG2 (see Supporting information, Appendix S1, Fig. S1). The selected landmarks provided a comprehensive sampling of the morphology of the biological structures under study (Zelditch et al., 2004).
Morphometric analysis of mandibles and skulls
The size of each mandible and skull was estimated as the centroid size (CS) obtained with TPSRELW and was transformed with natural logarithms. CS is a convenient estimator for size used commonly in geometric morphometric studies (Bookstein, 1996; Slice et al., 1996; Frost et al., 2003); it is uncorrelated with shape in the absence of allometry (Zelditch et al., 2004) and it is often highly correlated with body mass (Frost et al., 2003). The landmark configurations were aligned, translated, rotated, and scaled to unit CS using generalized Procrustes analysis, and the Procrustes coordinates and average landmark configuration were obtained (Rohlf & Slice, 1990). The Procrustes distances to the average configuration and pairwise Procrustes distances among samples (Zelditch et al., 2004) were computed, approximated to a Euclidean space using an orthogonal projection, and used as a measurement of morphometric distances.
The significance of allometry (change in shape associated with size differences) was tested for the continental and island groups separately for mandibles and skulls with multivariate regressions using MORPHOJ (Klingenberg, 2011). Allometry was significant in continental and island groups for mandibles and skulls; therefore, the regression slopes between groups were then compared with multivariate analysis of covariance in TPSREGR and were not statistically significant (data not shown) (Viscosi & Cardini, 2011). To control for allometric effects on mandible and skull shape variables, we performed multivariate regressions using MORPHOJ and kept the residuals as allometry-free shape variables for further analysis. We performed a principal components analysis (PCA) in JMP, version 10 (SAS Institute) on the shape variables and kept 16 and 17 PCs for mandibles and skulls, respectively, which explained ≥ 1% of total shape variation. We also carried out a variety of other preliminary analyses including landmark placement repeatability, sexual dimorphism, and a test for phylogenetic signal (see Supporting information, Appendix S1).
General ecogeographical patterns
For each specimen, we determined geographical data including latitudinal and longitudinal coordinates from fieldwork and museum records, as well as digital elevation data from the Consortium for Spatial Information at a 90 arc-min resolution (available at: http://srtm.csi.cgiar.org). Data for climatic variables (taken from the 1950–2000 period) were obtained from WorldClim (available at: http://www.worldclim.org) at a 2.5 arc-min resolution using DIVA-GIS, version 7.4.0.1 (http://www.diva-gis.org), including annual trends variables and extreme or limiting environmental variables: annual mean temperature (BIO1), maximum temperature of the warmest period (BIO5), minimum temperature of the coldest period (BIO6), annual precipitation (BIO12), precipitation of the wettest period (BIO13), precipitation of the driest period (BIO14), precipitation of the warmest quarter (BIO18), and precipitation of the coldest quarter (BIO19). Seasonal variables (annual range in temperature and precipitation) were excluded because they are composite climatic variables [e.g. BIO7 = temperature annual range (BIO5 − BIO6)] and would only complicate the interpretation of the results. We also obtained terrestrial net primary production (NPP) values from MODIS GPP/NPP (MOD17) at 1-km resolution from 2000 to 2009 (Zhao & Running, 2010). NPP is an environmental variable that quantifies the amount of atmospheric carbon fixed by plants and accumulated as biomass. In total, we obtained data for 12 geographical, climatic, and environmental variables and, for simplicity, they are referred to as ‘environmental variables’ throughout.
Because combinations of the 12 environmental variables showed correlations with each other, we performed a PCA using JMP on these variables and kept the first three environmental PCs for further analysis. PC1, PC2, and PC3 had eigenvalues ≥ 1.0 and together explained more than 80% of the variation for the environmental data sets (see Supporting information, Appendix S1, Tables S2, S3). The eigenvector matrices showed that: (1) PC1 was loaded with positive eigenvectors for all precipitation variables; low values indicate low precipitation mostly found not only in the central regions of the Iberian peninsula and eastern parts of the Balkan peninsula, but also in central–northern regions in Europe, whereas high values indicate high precipitation mostly found in the western coast of Ireland and in some areas of the Alps. (2) PC2 was loaded with a combination of negative eigenvectors for latitude and minimum temperature of the coldest period, and positive eigenvectors for longitude and altitude; low values indicate high latitude, low altitude, and moderate temperatures during winter mostly found in central and western regions of continental Europe and in the Atlantic islands, whereas high values indicate high altitude, low latitude, high longitude, and relatively low temperatures during winter mostly found in central and eastern regions, such as in the Balkan peninsula and in mountain areas of the Italian peninsula. (3) PC3 was loaded with a combination of negative eigenvectors for latitude and positive eigenvectors for annual mean temperature, maximum temperature of the warmest period, and NPP; low values indicate colder climate and moderate productivity from high latitudes, whereas high values indicate warmer climate and higher productivity mostly found in central latitudes.
Several statistical analyses were carried out on size and shape variables for the mandible and skull data sets. Using a standard least squares approach in JMP, we performed multiple regressions of size on latitude, altitude, and annual mean temperature (typical variables used to study Bergmann's rule) for the mandible and skull data sets. Because Bergmann's rule and the island rule may be better explored using biologically relevant environmental variables, we performed multiple regressions of size and shape on the three environmental PC for the mandible and skull data sets. This approach was used to examine the effects of each variable on size at the same time as controlling for the effects of the other variables. The significance of the models and of each variable was obtained with an analysis of variance comparing the fitted model with a simple mean model. Moreover, size differences between continental and island samples for the mandible and skull data sets were estimated with analysis of covariance (ANCOVA) in JMP using the three environmental PCs as covariates after testing for homogeneity of slopes.
To evaluate the environmental effects on mandible and skull shape, and to estimate how well the variation in shape can be predicted by environmental variables, we performed a multivariate multiple regression analysis of shape variables on the three environmental PCs using JMP. Two-block partial least squares analysis was conducted in JMP to describe the covariation between the geographical (latitude, longitude, altitude), climatic (WorldClim), and NPP variables with the variation in shape (see Supporting information, Appendix S1, Tables S4, S5). In two-block partial least squares analysis, linear combinations of the predictors are extracted with the objective of explaining as much of the variation in each response variable as possible, as well as accounting for variation in the predictors.
The mandible and skull photographs, landmark coordinates (in TPS format), and the environmental variables for all samples are available from DRYAD (doi:10.5061/dryad.cr1m5).
Genetic analysis
A total of 519 cytochrome b (cyt b) sequences of S. minutus were obtained from GenBank (AB175132, AJ535393–AJ535457, GQ494305–GQ494305, GQ272492–GQ272518, JF510321–JF510376). A sequence of Sorex volnuchini (AJ535458) from Anatolia was used as the outgroup (Fumagalli et al., 1999). DNA sequences were edited in BIOEDIT, version 7.0.9.0 (Hall, 1999) and aligned by eye. The phylogenetic relationships within S. minutus were inferred by Bayesian analysis sensu Vega et al. (2010a). The lineages found were the same as in previous phylogeographical studies (Mascheretti et al., 2003; McDevitt et al., 2010, 2011; Vega et al., 2010a, b) and were used as phylogroups for further analysis.
With DNASP, version 5.10 (Librado & Rozas, 2009), we calculated the corrected net number of nucleotide substitutions between pairs of phylogroups (DA), which represent the proportional sequence divergence among them (Nei, 1987). The pairwise divergence values (DA) among previously identified phylogroups were used for statistical comparison with the morphometric data. We used the matrix of pairwise DA values to construct a Neighbour-joining (NJ) tree with MEGA, version 4 (Tamura et al., 2007) to depict the evolutionary distances and relationships between the phylogroups.
Ecogeographical patterns in geographical groups
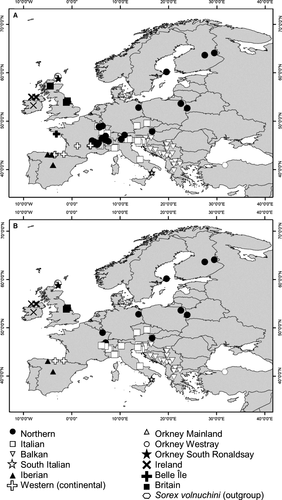
To analyze size and shape differences in S. minutus among regions in a phylogeographical context, we pooled the mandible and skull samples into 12 and 11 mutually exclusive geographical groups, respectively, according to their cyt b phylogroup membership (if DNA data were available from samples used in other studies) or to their known geographical origin (Fig. 1). The groups were designated as: ‘Iberian’, ‘Italian’, ‘South Italian’, ‘Balkan’, ‘Northern’, and ‘Western’. Island groups were identified separately as ‘Ireland’, ‘Orkney Mainland’, ‘Orkney Westray’, ‘Orkney South Ronaldsay’, ‘Belle Île’ (not available for skulls), and ‘Britain’.
We performed multiple regressions of size on the three environmental PCs using a standard least squares approach in JMP to determine the differences among the geographical groups at the same time as controlling for the effects of each predictor variable. Mandible and skull size differences among the groups were evaluated by ANCOVA followed by Tukey–Kramer post-hoc tests because this allows for unequal sample size (Sokal & Rohlf, 1995).
Mandible and skull shape differences among the groups were evaluated with multivariate analysis of variance (MANOVA) on the allometry-free shape variables (16 for mandibles and 17 for skulls), followed by Hotelling t2 tests for multivariate comparisons performed in PAST, version 2.17 (Hammer, Harper & Ryan, 2001). Shape changes were visualized as thin-plate spline transformation grids (Zelditch et al., 2004) computed with TPSSPLIN; available at: http://life.bio.sun ysb.edu/morph). Canonical variate analyses (CVA) using the shape variables as predictors were performed in JMP to differentiate among the groups for the mandible and skull data sets. The first two CVs were used to produce graphs for the samples showing separation by group membership (see Supporting information, Appendix S1, Table S6). Discriminant function analysis (DFA) was performed in JMP to estimate group membership of the mandible and skull data sets using linear combinations of the predictor variables that best discriminate between the groups. The leave-one-out (jackknife) with cross-validation approach was used to validate the DFA (Cardini et al., 2009). The results were averaged among three runs using a random subset of 70% of the samples from each group for training the model and 30% for testing. The number of discriminant functions used for analysis equalled the number of groups (K = 12 or K = 11) − 1.
The Procrustes distances among the average configurations of the groups (including the outgroup), for the mandible and skull data sets, were computed with TPSSMALL (available at: http://life.bio.sun ysb.edu/morph) and entered into PAST to produce distance matrices and distance trees using the NJ method to evaluate the morphological relationships. The geographical midpoints for the groups were calculated with the Geographic Midpoint Calculator (available at: http://www.geomidpoint.com/) and were used to obtain the pairwise geographical distances among them with the Geographic Distance Matrix Calculator, version 1.2.3 (by P. J. Ersts, available at: http://biodiversityinformatics.amnh.org/open_source/gdmg). Mantel tests were performed in PAST on pairwise Procrustes and geographical distances among the groups, as well as on pairwise Procrustes distances among the groups and pairwise genetic divergence (DA) values of the cyt b phylogroups. In addition, we performed a partial Mantel test of Procrustes distances and geographical distances but controlling for genetic distance. The significance of the tests was obtained by a permutation procedure with 10 000 bootstraps. Mandible and skull CS and Procrustes distances were mapped onto the NJ tree of cyt b phylogroups using squared-change parsimony in MESQUITE, version 2.75 (Maddison & Maddison, 2011) to show size and shape evolution using eight categorical bins.
Results
General ecogeographical patterns
The results from multiple regressions of size on latitude, altitude, and annual mean temperature), or on environmental variables (PC1, PC2, and PC3), are summarized in Table 1 (see also Supporting information, Appendix S1, Table S3). Typical Bergmann's rule variables statistically predicted mandible size, although the data contain a high amount of unexplained variability (F4,563 = 5.274, P < 0.001, r2 = 0.036). Latitude was negatively related with size, and annual mean temperature did not contribute significantly to the model. Environmental variables statistically predicted mandible size also with a high amount of unexplained variability (F4,563 = 4.179, P = 0.02, r2 = 0.029). All variables were positively related with size and contributed significantly to the model. On average, continental samples showed significantly larger mandible size than island samples (F = 6.204, P = 0.013), mostly driven by the larger mandible size of southern samples from continental Europe. Typical Bergmann's rule variables statistically predicted skull size, and the model explained more variability compared to the mandible data set (F4,372 = 31.155, P < 0.001, r2 = 0.251). Annual mean temperature did not contribute significantly to the model and latitude only marginally so. Environmental variables statistically predicted skull size with a high amount of unexplained variability (F4,372 = 4.1, P = 0.03, r2 = 0.042) and only PC1 contributed significantly to the model. On average, island samples showed marginally significant larger skull size than continental samples (F = 4.661, P = 0.031).
| Traditional Bergmann's rule variables | Geographical and environmental variables | ||||||
|---|---|---|---|---|---|---|---|
| Factor | Coefficienta | t-valueb | P-value | Factor | Coefficient | t-value | P-value |
| Mandibles (N = 568) | |||||||
| Latitude | −0.002 | −6.723 | < 0.001 | PC1 | 0.008 | 9.211 | < 0.001 |
| Altitude | 0.000 | 7.022 | < 0.001 | PC2 | 0.003 | 2.501 | 0.013 |
| Annual mean temperature | 0.001 | 1.427 | 0.154 | PC3 | 0.005 | 3.959 | < 0.001 |
| Factor | Coefficient | t-value | P-value | Factor | Coefficient | t-value | P-value |
|---|---|---|---|---|---|---|---|
| Skulls (N = 377) | |||||||
| Latitude | 0.000 | −1.975 | 0.049 | PC1 | 0.002 | 3.303 | 0.001 |
| Altitude | 0.000 | 3.066 | 0.002 | PC2 | −0.001 | −1.617 | 0.107 |
| Annual mean temperature | 0.001 | 1.379 | 0.169 | PC3 | 0.000 | −0.266 | 0.790 |
- a Unstandardized coefficients.
- b Test for the statistical significance of each independent variable.
Environmental variables had small but significant effects on allometry-free shape of mandibles and skulls, and together accounted for 5.1% and 11.9% of mandible and skull shape variation, respectively (Table 2). PC3 explained the highest percentage of shape variation in both data sets. With the two-block partial least squares analysis, 10 and nine factors were extracted that explained 13.6% and 18.4% of mandible and skull shape variation, respectively (see Supporting information, Appendix S1, Tables S4, S5).
| All factors | PC1 | PC2 | PC3 | |
|---|---|---|---|---|
| Mandibles | ||||
| Wilk's λ | 0.240 | 0.792 | 0.653 | 0.613 |
| F ratio | 7.139 | 4.387 | 8.895 | 10.556 |
| DF1 | 128 | 32 | 32 | 32 |
| DF2 | 2119 | 535 | 535 | 535 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Percentage explained | 5.1 | 0.9 | 1.7 | 2.4 |
| Skulls | ||||
| Wilk's λ | 0.1583 | 0.7748 | 0.5284 | 0.5697 |
| F ratio | 5.8560 | 2.9230 | 8.9760 | 7.5960 |
| DF1 | 136 | 34 | 34 | 34 |
| DF2 | 1352 | 342 | 342 | 342 |
| P-value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Percentage explained | 11.9 | 0.92 | 4.8 | 6.2 |
- DF, degrees of freedom.
Genetic analysis
There were 303 cyt b haplotypes for S. minutus that clustered into six main phylogroups (Mascheretti et al., 2003; McDevitt et al., 2010, 2011; Vega et al., 2010a, b). We distinguished the following continental phylogroups for comparison with the morphological data: ‘Northern’ (N = 101), which included samples from Central and Northern Europe to Lake Baikal in Siberia. ‘Italian’ (N = 26), mostly restricted to the northern and central parts of the Italian peninsula. ‘Western’ (N = 15), which included samples from the Cantabrian Mountains, the Pyrenees, and Western France. ‘South Italian’ (N = 4), geographically restricted to La Sila Mountain, Calabria, in Southern Italy. ‘Iberian’ (N = 3), geographically restricted to the Iberian peninsula. ‘Balkan’ (N = 4), which included samples from Macedonia and Turkish Thrace in the Balkan peninsula. We also distinguished the following island groups: ‘Ireland’ (N = 94), ‘Orkney Mainland’ (N = 44), ‘Orkney Westray’ (N = 33), ‘Orkney South Ronaldsay’ (N = 40), and ‘Belle Île’ (N = 5), which clustered within the Western clade, and ‘Britain’ (N = 91), which clustered within the Northern clade. Other samples (N = 59) clustered in the Western clade in the molecular studies but were not used in the present study because they belong to islands in the periphery of Britain from where there were no morphological samples for comparison. Pairwise divergence (DA) values among the phylogroups are shown in the Supporting information (Appendix S2, Tables S7, S8). The South Italian, Iberian, and Balkan groups and the outgroup showed the highest pairwise DA values, whereas pairwise DA values among the Western, Irish, and Orkney islands groups were the lowest.
Ecogeographical patterns in geographical groups
When controlling for environmental factors, we found significant size differences among groups for the mandible and skull data sets (mandibles: F11,556 = 24.186, P < 0.001; skulls: F10,366 = 8.658, P < 0.001; see Supporting information, Appendix S3, Table S9).
For mandible and skull size, there were latitudinal trends converse to Bergmann's rule among the continental groups, and island effects for the island groups (Fig. 2). The South Italian, Iberian, and Balkan groups, belonging to the southernmost latitudes, had the largest mandibles among the continental groups. The Northern group had the smallest mandible of all continental groups and was also significantly different from all other continental groups but not significantly different from some island groups. The Orkney Mainland group, although at a high latitude, had the largest mandible of all island groups, whereas it was only significantly different from Orkney South Ronaldsay. All other island groups had comparable mandible sizes to those found in continental groups but larger than expected by latitude. The skull data set showed less variation in size among the groups than the mandible data set, whereas it had a decreasing size tendency with increasing latitude. The Iberian group had the largest skulls of the continental samples. The Northern group had the smallest skulls on average, as in the mandible data set, although this group was only significantly different in size from the Iberian and Orkney Westray groups. Notably, the skulls from the Orkney islands were as large as those from the southern groups and larger than those from the northern group, indicative of an island effect even controlling for the latitudinal effect. The results relating to South Italy and Britain should be interpreted with caution because of the low sample size, although they are still indicative of the size trends in these two areas.
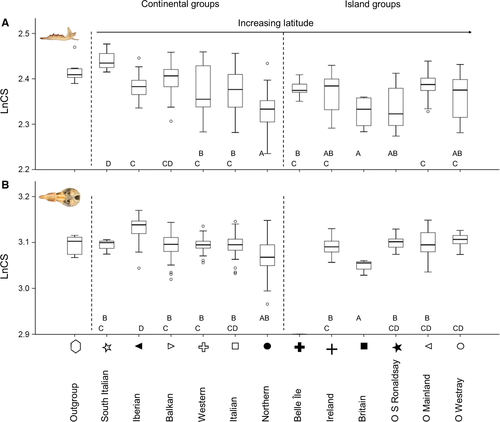
The MANOVA on allometry-free shape variables of mandibles and skulls showed significant differences among the groups (mandibles: Wilks’ λ = 0.1954, F176,4959 = 5.521, P < 0.001; skulls: Wilks’ λ = 0.0415, F170,3056 = 5.319, P < 0.001; see Supporting information, Appendix S3, Tables S10, S11). Based on thin-plate splines (Fig. 3), shape variation was small and mostly evident between the southern groups and the Orkney islands. In southern latitudes and in larger mandibles, there was a relative forward movement of the landmarks on the lower part of the mandible (landmarks 1 and 16–18) in relation to the landmarks between teeth alveoli (landmarks 3–8), and a relative forward shift of the coronoid process (Fig. 3A). The three groups from the Orkney islands had notable backward shifts of the coronoid process compared to the other groups, with Westray also showing pronounced variation in the frontal part of the mandible, whereas, in the Iberian and Balkan groups, the coronoid process moved slightly forward (Fig. 3A). In southern latitudes and in larger skulls, there was an outward movement of landmarks 2 and 7 in relation to other landmarks between teeth alveoli (landmarks 3–6, 8, and 9), and opposite movements of landmarks 16 and 17 (Fig. 3B). This generally resulted in a wider separation of the upper premolars, less pointed snouts, and smaller foramen magnum compared to skulls from northern latitudes (Fig. 3B).
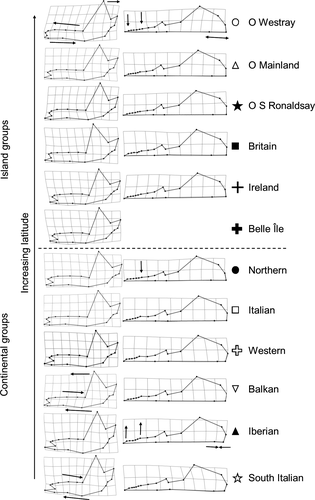
The first two CVs explained 69.6% and 62.2% of total shape variation among groups in the mandible and skull data sets, respectively (see Supporting information, Appendix S1, Table S6). For purposes of visualization, scatter plots of the first two CVs are presented with group memberships for mandibles (Fig. 4A) and skulls (Fig. 4B). In both data sets, the shape distribution of the continental groups mostly overlapped, whereas Ireland and the Orkney islands could be discriminated. Westray was the island group that was most easily discriminated, in accordance with the large Procrustes distances and shape variation found in the mandible and skull data sets. Belle Île (mandible data set only) and Britain (mandibles and skull data sets) could not be differentiated from the continental samples. With the DFA, on average, we correctly classified 44.9% and 54.6% of the individuals to their predefined group of mandibles and skulls, respectively; however, this was mostly a result of the low classification scores for the continental groups. The classification scores in the mandible and skull data sets were high the Orkney islands groups in agreement with its notable shape differences.
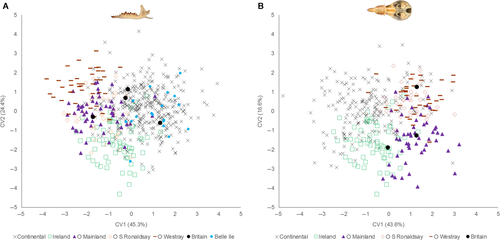
There were different topologies among the phylogenetic tree and the Procrustes distances trees of mandibles and skulls (Fig. 5). For mandible and skull shape, the South Italian group is the first to split from the rest, and Orkney Westray shows the highest shape distance of all groups (Fig. 5A, B). Intraspecific variation in size and shape, mapped using squared change parsimony and visualized on the NJ tree of phylogroups (based on DA), showed no apparent relationship of size and shape with phylogenetic history of S. minutus (Fig. 5C, D, E, F). The Mantel tests revealed that there were significant positive correlations between Procrustes and geographical distances of mandible (r = 0.2653, P = 0.0471) and skull groups (r = 0.6019, P = 0.0004). However, the correlations between Procrustes and genetic distances were not significant for mandible (r = −0.0827, P = 0.5978) and skull groups (r = −0.2189, P = 0.8869). When controlling for genetic distances, partial Mantel tests also revealed significant correlations among Procrustes and geographical distances for mandible (r = 0.2935, P = 0.0360) and skull groups (r = 0.6818, P < 0.0001). Pairwise geographical and Procrustes distances among mandible and skull groups are shown in the Supporting information (Appendix S2, Tables S7, S8).
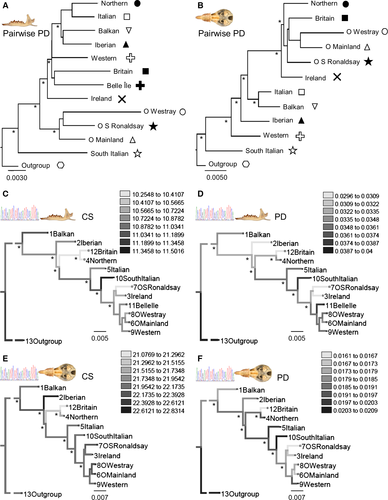
Discussion
Continental differentiation in S. minutus
Bergmann's rule has traditionally been studied in terms of latitude, altitude, and temperature (Meiri & Dayan, 2003; Meiri, 2011) and this was explored in the present study in S. minutus. However, because Bergmann's rule may relate to a combination or an interaction of environmental factors, we also explored the morphological variation in S. minutus in relation to a whole range of geographical, climatic, and NPP variables within a phylogeographical and continental-and-island framework.
For S. minutus, the significant negative relationship of mandible size with latitude, as well as the larger mandible and skull size in southern compared to northern continental groups, indicates a pattern converse to Bergmann's rule. Using PC of geographical and environmental variables shows a more complex basis to the size trends in S. minutus rather than simply an impact of latitude, altitude or temperature. PC1, PC2, and PC3, loaded with various combinations of latitude, longitude, temperature, and precipitation variables, as well as NPP, consistently showed a positive relationship with mandible size, although only PC1 showed a positive relationship with skull size. We concur with McNab (2010) that an emphasis in relation to Bergmann's rule may be unhelpful, and that the size trends relate to the availability of resources in a broad sense, which in turn relates to various underlying environmental factors.
The converse Bergmann's rule has frequently been reported in shrews and may be a common trend within Soricidae; for exceptions, see White & Searle (2007) who found Bergmann's rule in Sorex araneus from British islands, and Ochocińska & Taylor (2003) who showed nonsignificant relationships of size with latitude for Sorex isodon and Sorex tundrensis. Accordingly, the condylobasal skull lengths of S. araneus, S. caecutiens, and S. minutus from the Palearctic region relate negatively to latitude (Ochocińska & Taylor, 2003). Three mainland populations of Sorex trowbridgii from Western USA have decreasing cranial and mandibular dimensions with increasing latitude (Carraway & Verts, 2005) and variation in body size of Sorex cinereus in Alaska contradicts Bergmann's rule (Yom-Tov & Yom-Tov, 2005). Morphological measurements of Neomys anomalus from Eastern Europe and the Balkans also relate negatively to latitude but show evidence of character displacement when in sympatry with Neomys fodiens (Kryštufek & Quadracci, 2008). The northern short-tailed shrew (Blarina brevicauda) has a negative, albeit nonsignificant relationship of size with latitude (Ashton et al., 2000). Consistent with converse Bergmann's rule, size in N. anomalus and N. fodiens from Poland was the smallest in the north and largest in the south when in sympatry but, when in allopatry, both species were larger at northern latitudes, showing the opposite pattern (Rychlik, Ramalhinho & Polly, 2006).
Regarding shape patterns, environmental variables (reflected in the first three PCs) explained small percentages of total shape variation (5.1% and 11.9% for mandibles and skulls, respectively). It is not surprising that so much shape variation remained unexplained because other exogenous and endogenous factors may be playing important roles. Based on the CVA, evolution on islands maybe a contributing factor. In a similar ecogeographical study on the primate Cercopithecus aethiops from sub-Saharan Africa, the response of skulls to climatic variables was stronger for size than for shape despite the evident intraspecific geographical differences, and approximately 80% of shape variance remained unexplained (Cardini, Jansson & Elton, 2007). Morphology can also be influenced in a complex way by climatic and phylogenetic factors and, in Microtus savii, both sets of factors contribute to shape variation of the first lower molars, whereas tooth size is not affected by climatic conditions (Piras et al., 2010). However, we did not detect a significant phylogenetic signal and the mapping of size and shape on the phylogeny showed no apparent relationships. Although Mantel tests showed no relationships of shape and genetic distances, the results have to be interpreted with caution because Mantel test has lower power in comparison with other tests (Legendre & Fortin, 2010); however, the Mantel test has been traditionally used in morphological, ecological, and genetic studies, it is useful when data can be expressed as distances, and the results are coherent with the other results reported in the present study.
Why is the pygmy shrew generally smaller in northern latitudes compared to southern latitudes? There is some dispute about the mechanisms involved for Bergmann's rule or its converse (Blackburn et al., 1999; Meiri, 2011). However, the lower food availability in northern, colder or less productive habitats is likely to be a selective factor acting on small mammals, combined with lower absolute food requirements for smaller vs. larger species of small mammals in less productive habitats (Ochocińska & Taylor, 2003). This may explain the small size of shrews of the northern group of S. minutus, which evolved in and expanded from northern glacial refugial areas (Vega et al., 2010a). Populations of S. araneus in Finland are up to 13% smaller inland than at the coast, with the main differences being lower winter temperatures and less snow cover at inland sites, which are factors associated with a lower habitat productivity that could selectively favour smaller shrews (Frafjord, 2008). In S. cinereus, it has been suggested that the increase in size during the second half of the twentieth century is related to increasing winter temperatures and higher food availability in winter as a result of improved weather conditions for its prey (Yom-Tov & Yom-Tov, 2005).
Dehnel's phenomenon (i.e. a reduction of body size and the mass of organs of soricine shrews from northern temperate regions during winter) has been interpreted as an adaptation to reduced prey abundance permitting a reduction in absolute food requirements in a group of species that do not hibernate. However, recent findings indicate that prey numbers and biomass available for shrews (which do not hibernate) do not decrease during winter, whereas soil invertebrates do change their vertical distribution, apparently requiring shrews to have a modified, more energetically costly foraging behaviour for the consumption of energetically less favourable prey (Churchfield, Rychlik & Taylor, 2012). In the present study, a Dehnel effect is unlikely to play a role because < 5% of our samples were collected during winter (i.e. those few individuals that were collected in winter were from Switzerland where we have a good sample size, as well as from Central Spain where the results indicate large mandible and skull size). It should be noted that phenotypic plasticity (i.e. the ability of a single genotype to produce more than one alternative form of morphology, physiological state or behaviour in response to changes in environmental conditions) cannot be ruled out as a possible explanation until proper experimental studies are undertaken with shrews (Husby, Hille & Visser, 2011).
Size and shape variation of the mandible can affect the biomechanics of mastication by modifying the sites of attachment of mandible muscles (Monteiro, Duarte & dos Reis, 2003). Larger and morphologically distinctive mandibles could reflect a stronger bite force or a higher mechanical potential for mastication, which could be an adaptation or a plastic response to more arid conditions, allowing exploitation of a wider size-range of prey and prey with harder exoskeletons, and/or character release in the absence of competitors (Strait, 1993; Monteiro et al., 2003; Carraway & Verts, 2005). The association of diet and skull shape can be strong because muscles used for mastication are tightly linked to bone structure; for example, diet may explain up to 25% of skull shape variance in marmots (Caumul & Polly, 2005). In S. minutus, a stronger bite force was estimated for South Italian than for north European populations in relation to the positioning of the coronoid process and horizontal ramus length (Vega et al., 2010b), and the morphological patterns described in that study were similar to those reported in the present study.
Island differentiation in S. minutus
Under the island rule, it is expected that small mammals on islands will have a larger body mass than mainland conspecifics (Van Valen, 1973). Our results indicate that there is a strong island effect operating on the size of mandibles and skulls of S. minutus from Ireland and the Orkney islands. Moreover, these island groups were distinctive from continental groups in terms of shape variation, and samples were assigned correctly to their island of origin. There was a lack of correspondence between Procrustes distances and cyt b tree terminal branches. Overall, it appears that environmental factors and insularity have stronger effects on morphology, perhaps through local adaptation, genetic bottlenecks, and/or plastic responses, than those provided by phylogenetic relationships. Therefore, S. minutus from Ireland and the Orkney islands shows morphological differentiation from continental groups through island effects, whereas cyt b reveals the close phylogenetic relationship of these island groups with continental Western Europe (McDevitt et al., 2011).
Other shrew species on islands share similar trends. For example, S. trowbridgii from Destruction Island (Washington State, USA) has greater average skull-breadth and mandibular dimensions than the mainland counterpart (Carraway & Verts, 2005). Sorex araneus from several Scottish islands are significantly larger than populations in mainland Britain and show larger body size on islands in relation to distance to the mainland (White & Searle, 2007). Crocidura russula from several French islands also shows divergence in mandible shape in relation to distance from the mainland and island size (Cornette et al., 2012). Crocidura suaveolens from Corsica is larger and has a smaller litter size than mainland populations in Southern France (Fons et al., 1997), indicating an island effect (Adler & Levins, 1994). Studies of other small mammals have also shown morphological divergence of recently colonized island populations (Michaux et al., 2007; Renaud & Michaux, 2007; Cucchi et al., 2014). Similar to the present study, the mandible and skull shape of Marmota vancouverensis from Vancouver Island is highly divergent from the mainland counterpart Marmota caligata, despite small mtDNA sequence divergence (Cardini, 2003; Cardini & O'Higgins, 2004). Previous morphological studies on S. minutus from islands around Britain relate to presence/absence of S. araneus (Malmquist, 1985), although the results are difficult to interpret because of anomalies in the reporting of sympatric and allopatric status of S. minutus on these islands.
It may be possible that morphological traits in mammals evolve quickly on islands in a matter of a few decades after colonization (Pergams & Ashley, 2001; Millien, 2006; Cucchi et al., 2014; but see also Meiri et al., 2006, 2008; Raia & Meiri, 2006, 2011). Given that S. minutus is the only shrew species in the Orkney islands and, until recently, it was the only shrew species in Ireland, a larger body mass (reflected in larger mandibles and skulls) could have evolved on these islands driven by competitive release, the absence of predators, and the availability of resources (McDevitt et al., 2014). Additionally, geographical isolation from continental populations for several thousand years, genetic bottlenecks after colonizations from a low number of migrants, and low genetic diversity (i.e. very few cyt b haplotypes were observed in the Orkney islands despite the large sample size) could lead to deviation in the morphology of island populations of S. minutus compared to the mainland (Cornette et al., 2012). By contrast, specimens of S. minutus in Belle Île and Britain have higher cyt b diversity (McDevitt et al., 2011) and are similar in terms of mandible shape to continental samples. Additionally, Belle Île and mainland Britain are occupied by other species of shrews.
Morphological differences may actually represent phenotypic plasticity expressed in insular environments; however, this hypothesis has rarely been tested. However, with our results in S. minutus, we cannot rule out phenotypic plasticity as a possible explanation; at least for C. suaveolens, differences in body size and litter size between island and mainland populations were persistent over three generations under laboratory breeding conditions, thus supporting the hypothesis that these differences are genetically determined rather than a result of phenotypic plasticity (Fons et al., 1997). The evolution of different size and shape in island populations of S. minutus may thus be an adaptive response to changed availability of resources, the ‘resource rule’ sensu McNab (2010), acting together with demographic and historical factors.
Conclusions
In the present study, we have explored the morphological variation of mandibles and skulls of S. minutus across Europe using a geometric morphometric approach. We found notable ecogeographical variation in mandible and skull size related to environmental variables and insularity, which may suggest that the converse Bergmann's rule and the island rule operate in S. minutus. However, we consider that these ecogeographical patterns could be more reasonably explained as a response to resource availability, possibly reflecting adaptation or a phenotypically plastic response to different habitats and environmental conditions, differential allocation of energy and physiological responses, differential food availability, and the presence/absence of competitors. Correlative studies such as the present one are an important means of identifying patterns that require further investigation by in-depth studies measuring the strength of selection or the experimental link between performance, morphology, and ecology generating local adaptations (Calsbeek & Irschick, 2007).
Considering variation in morphological shape rather than size, the most divergent populations among those examined in S. minutus were from the Atlantic islands, although distinctive features could also be identified for populations in southern Europe (e.g. with thin-plate spline transformation grids). Interestingly, with respect to both size and shape, the morphological variation observed in the present study does not follow previous genetic subdivisions within the species, and instead indicates a complex role for different evolutionary and/or environmental processes in determining geographical variation in S. minutus.
Acknowledgements
Specimens of Sorex minutus were made available by several museums and we acknowledge the help from the following institutions: Institut de Zoologie et d’Écologie Animale (Université de Lausanne, Switzerland), Slovenian Museum of Natural History, Mammal Research Institute of the Polish Academy of Sciences (Białowieża, Poland), Museo di Anatomia Comparata and Museo di Zoologia ‘La Sapienza’ (Università di Roma, Italy), Museo di Storia Naturale ‘La Specola’ (Firenze, Italy), Museo Civico di Storia Naturale Giacomo Doria (Genova, Italy), and Dipartimento di Valorizzazione e Protezione delle Risorse Agroforestali (Università degli Studi di Torino, Italy). We are very grateful for the samples provided by Heidi C. Hauffe, Patrick Brunet-Lecomte, Jan Wójcik, Enrique Castiens, Sandro Bertolino, Giuliano Doria, Paolo Agnelli, and Giovanni Amori. Paul O'Higgins provided important theoretical insights during the development of this project and Ardern Hulme-Beaman helped with statistical advice. We thank three anonymous reviewers for their helpful comments. RV received funds from CONACyT (Mexico; Reg. No. 181844) and ADM received funds from the Irish Research Council.
References
Shared Data
Data available from the Dryad Digital Repository: doi:10.5061/dryad.cr1m5 (Vega R et al., 2016).




