Patterns in the diversity and endemism of extant Eocene age lineages across southern Africa
Abstract
Southern Africa boasts a wealth of endemic fauna and flora, comprising both massive recent radiations such as those characteristic of the Cape flora, and solitary ancient species such as the peculiar desert gymnosperm Welwitschia. This study was undertaken to identify ancient biological lineages (tetrapod and vascular plant lineages of Eocene age or older) endemic to southern Africa, and to map their distribution across the region. Twenty-seven (17 plant and ten animal) lineages were identified, and distribution maps were generated for each of them across 74 operational geographic units, which were then combined into total endemism and corrected weighted endemism per unit area. Total endemism peaked along South Africa's coast and Great Escarpment, but in the case of weighted endemism high values were also recorded along other portions of the Great Escarpment further north. A review of the lineages sister to southern African ancient endemic lineages showed that these are often globally widespread, and many of them differ substantially from the southern African ancient lineages in terms of morphology and ecology. The mechanisms of ancient lineage survival in the region are discussed, and their importance for conservation in southern Africa is emphasised.
Introduction
In today's rapidly changing world, the need for prioritising biodiversity conservation efforts is evident (Hooper, Kennedy & Quinn, 2002; Cowling et al., 2003; Isaac et al., 2007), for which species diversity alone is increasingly viewed as insufficient. The evolutionary history of species is also an important component of biodiversity, taking into consideration the historical and ecological processes resulting in speciation and evolution. The loss of old monotypic lineages will result in a greater loss of biodiversity as opposed to species losses in speciose lineages (Purvis et al., 2000; Sechrest et al., 2002; Forest et al., 2007; Isaac et al., 2007; Rosauer et al., 2009; Tucker & Cadotte, 2013).
Phylogenetic diversity and phylogenetic endemism are two of the measures employed to incorporate the increased value of preserving ancient; and thus more distinctive, species. Phylogenetic diversity (PD) is a biodiversity measure which uses the branch lengths of the evolutionary pathways that connect a selected set of taxa, and hence can identify sets of taxa that maximise feature diversity (Faith, 1992; Forest et al., 2007). Phylogenetic endemism (PE) is a combination of PD and weighted endemism, used to highlight areas where an important proportion of PD is exclusively found (Rosauer et al., 2009). These measures have been recently used in mapping studies focused on specific groups of plants and animals globally or regionally (amphibians – Fritz & Rahbek, 2012; mammals – Rosauer & Jetz, 2014; plants – Costion et al., 2014). Although widely used, these measures still remain, to some extent ‘black boxes’, in that they combine ancient lineages and recent radiations into a single value for one given area.
The southern African region provides a good context to study the importance of ancient evolutionary lineages, as this region is not only rich in ancient lineages, but also in recent radiations, such as those of the Cape Flora. The unique combination of relative geomorphological, tectonic and climatic stability, as well as the high levels of total biodiversity and endemism, gives rise to the potential development of evolutionary refugia (Brooks et al., 2015). Here we focus on ancient lineages endemic to southern Africa, to emphasise their potential for directing further conservation planning and implementation efforts in this region. Furthermore, we contrast ancient endemic lineages with their sister lineages to emphasise their distinctiveness, and some potential factors relevant to their persistence.
Material and Methods
Study region
Southern Africa, comprising South Africa, Lesotho, Swaziland, Namibia, Zimbabwe, Zambia, Angola, Malawi and Botswana, covers an area of approximately 6 million km2. In addition, five of the world's global biodiversity hotspots are found within this region: the Cape Floristic Region (CFR), Succulent Karoo (SK), Maputaland–Pondoland–Albany (MPA), parts of the Eastern Afromontane (EA) and the Coastal Forests of Eastern Africa (CFEA) (Myers et al., 2000; Mittermeier et al., 2004). The CFR is also regarded as one of the six floral kingdoms of the world (Goldblatt, 1978; Burgess, 1998; Linder et al., 2010).
Selection of lineages and distribution mapping
We used an age limit of 30 Myr, roughly coinciding with the Eocene–Oligocene transition (Zachos et al., 2001; Liu et al., 2009). The transition between the Eocene and Oligocene was marked by substantial changes in the world's climate, relevant to the survival of ancient lineages (Zachos et al., 2001; Tsubamoto, Takai & Egi, 2004). We used the stem ages of the broadest lineages endemic to the study region as lineage age values. We then reviewed distribution data from the literature to determine which lineages were endemic to southern Africa, and consequently we set a latitudinal limit to 10°28′S, only considering lineages endemic to the region south of this latitude. This ensured that East African endemics were excluded, while still retaining southern Africa in its broadest sense. However, we only mapped the distributions of these lineages south of 14°45′S (based on Operational Geographic Unit limits, see below). This difference created a buffer where some lineages endemic to southern Africa occur but are not mapped, and was meant to ensure that any effect of decreasing endemism towards the north is not simply a boundary artefact. OneZoom (Rosindell & Harmon, 2012), an online engine providing age estimates for all tetrapod vertebrates and seed plants, was used to identify ancient biological lineages endemic to southern Africa. As the age values for some squamate lineages did not appear reliable in this engine, we identified ancient lineages in this group from published papers, two gecko lineages (Rhoptropella and Narudasia) meeting the age criterion as derived from the work of Gamble et al. (2011).
Operational Geographic Units (OGUs) in the present study were modified based on such units by Born, Linder & Desmet (2007) for the Cape region, and Perera, Ratnayake-Perera & Procheş (2011) for the eastern region of southern Africa (Supporting Information, Fig. S1, OGU map; Table S1, OGU names and codes). Where OGUs were not available, we created OGUs by merging Quarter Degree Square (QDS) cells to form single units, roughly coinciding with vegetation units present in southern Africa (Sayre et al., 2013). Distributional data are easily accessible at QDS level for some of the lineages, simplifying the mapping process. The OGU scale was chosen as this minimised the errors of omission which would have occurred if using a finer scale (such as QDS units; see Perera et al., 2011 – the OGUs used in this study contained an average of 77 ± 52 QDSs).
Several endemism measures were used. Initially, the sum total of the selected lineages present within each OGU was calculated to give the total lineages present (Crisp et al., 2001; ‘total endemism’ hereafter); this allowed for the creation of a comprehensive map of the diversity of ancient endemic lineages. As some lineages are more widespread than others, these can be defined as board or narrow endemics based on the number of OGUs they occupy. However, this manner of defining lineages place arbitrary limits to endemism (Crisp et al., 2001). To avoid these arbitrary limits, Crisp et al. (2001) re-introduced a measure called ‘weighted endemism’ (Dony & Denholm, 1985; Williams, Humphries & Gaston, 1994).
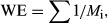 (1)
(1) (2)
(2) (3)
(3)ESRI ArcGIS 9.2 (ESRI, 2006) was used to create all maps produced in this study. All statistical analyses were performed using R 3.1.0 (R Core Development Team, 2014; available at: http://www.r-project.org/).
The online engine ‘OneZoom’ (Rosindell & Harmon, 2012) was also used to identify the sister lineages of the selected ancient endemic lineages of southern Africa. We reviewed the literature to determine the distribution, habitat, and broad morpho-ecological characteristics (‘life forms’ for plant lineages), relevant to the ecological niche of the ancient endemic lineages and sister lineages. In this manner, similarities and differences of ancient endemic lineages of southern Africa with their sister lineages were used to emphasise the uniqueness of southern African endemic ancient lineages, and their possible biogeographic links.
Results
Seventeen plant lineages and ten animal lineages, consisting of six reptiles, two mammals, one bird, and one amphibian lineage, met the age and endemism criteria (see Fig. 1, for examples of selected lineages; Table 1). Individual lineage maps for all lineages are presented in Supporting Information (Figs S2–S4).

| Lineage: | Class: | Age (Myr): | Number of species: | Presence (no. OGUs): | |
|---|---|---|---|---|---|
| 1. | Heleophrynidaea | Amphibia | 34 | 7 | 12 |
| 2. | Bradypodion a | Reptilia | 49 | 18 | 23 |
| 3. | Platysaurus a | Reptilia | 49.5 | 15 | 27 |
| 4. | Afroedura a | Reptilia | 111.7 | 13 | 37 |
| 5. | Rhoptropus (Gamble et al., 2011) | Reptilia | ±55 | 11 | 9 |
| 6. | Rhoptropella (cf. Gamble et al., 2011) | Reptilia | ±50 | 1 | 3 |
| 7. | Narudasiaa (Gamble et al., 2011) | Reptilia | ±80 | 1 | 8 |
| 8. | Malacothrix a | Mammalia | 48 | 1 | 33 |
| 9. | Petromus a | Mammalia | 45.3 | 1 | 15 |
| 10. | Promerops a | Aves | 40 | 2 | 22 |
| 11 | Stangeria a | Cycadopsida | 83.4 | 1 | 5 |
| 12. | Welwitschia a | Gnetopsida | 87.1 | 1 | 10 |
| 13. | Bruniaceaea | Eudicotida | 76.2 | 75 | 10 |
| 14 | Geissoloma a | Eudicotida | 53.7 | 1 | 2 |
| 15. | Greyia a | Eudicotida | 41.1 | 3 | 13 |
| 16. | Grubbia + Curtisiaa | Eudicotida | 91.9 | 6 | 30 |
| 17. | Roridula a | Eudicotida | 70.2 | 2 | 2 |
| 18. | Moringa ovalifolia a | Eudicotida | 48.6 | 1 | 12 |
| 19. | Nectaropetalum a | Eudicotida | 60.2 | 5 | 3 |
| 20. | Grielum a | Eudicotida | 35.4 | 4 | 13 |
| 21. | Hypocalyptus a | Eudicotida | 37.5 | 3 | 3 |
| 22. | Achariaeaea | Eudicotida | 31.8 | 6 | 16 |
| 23. | Lanaria a | Monocotylenopsida | 39.8 | 1 | 4 |
| 24. | Agapanthus a | Monocotylenopsida | 46.7 | 4 | 17 |
| 25. | Anthochortus + Willdenowiaa | Monocotylenopsida | 35.8 | 19 | 5 |
| 26. | Tulbaghia a | Monocotylenopsida | 48.7 | 26 | 45 |
| 27. | Nivenioideaea | Monocotylenopsida | 51.7 | 15 | 3 |
- a OneZoom (see Rosindell & Harmon, 2012).
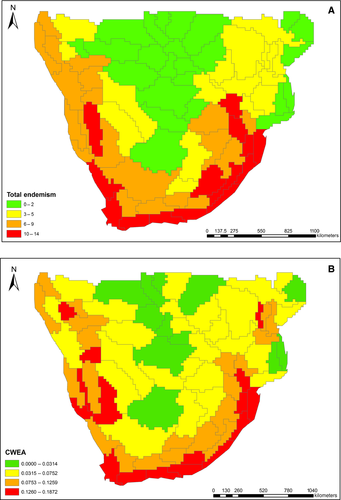
The highest total endemism scores were found along the coastal regions of southern Africa, as well as along parts of the Great Escarpment (Fig. 2A, indicated in red). Total endemism decreased northwards and towards inland regions (Fig. 2A). Fig. 2B shows the CWEA for southern Africa. The highest values, again, were found along the coastal regions of southern Africa. Similar to total endemism, CWEA shows a decreasing pattern of endemism towards inland regions, although there were localised high values in the highlands of Angola and mountainous areas of Namibia and Zimbabwe.
When comparing the ancient southern African endemic lineages with their sister lineages, many sister lineages were shown to have widespread distributions across the world (Table S2, Supporting Information). Life form differences were noted between eight of the ancient plant lineages endemic to southern Africa (Grubbia + Curtisia, Geissoloma Greyia, Roridula, Stangeria, Welwitschia, Achariaceae and Nivenioideae) and their sister lineages (Table S2, Supporting Information). Five ancient lineages (Bruniaceae, Geissoloma, Greyia, Roridula, Welwitschia, Moringa ovalifolia) were found to occupy different habitats in comparison with their sister lineages (Table S2, Supporting Information).
Discussion
Many recent studies highlight southern Africa as a whole as having a wealth of accumulated evolutionary history, as can be seen on global phylogenetic diversity and phylogenetic endemism maps (Fritz & Rahbek, 2012; Procheş & Ramdhani, 2013; Rosauer & Jetz, 2014), or even on maps of lineage diversity for lineages of a given age, as done regionally in this study and globally, though in only one group, by Davies & Buckley (2011). This wealth can be explained by considering the fact that, through the climatic fluctuations of the Cenozoic, Africa did not migrate substantially across latitudinal belts, and its climate may have been stable when compared to other parts of the world. Further evidence for climatic stability in southern Africa is provided by Dynesius & Jansson (2000), albeit over a shorter time period than the one considered here. Additionally, Altwegg et al. (2014) predicted relatively stable future climatic conditions for at least some parts of the region. Thus, while southern Africa had already been shown to be a repository of ancient lineages and some explanations were readily available, the scale of these observations was coarse, and the taxonomic breadth limited. Our study refines this by detailing patterns within the region, but also by expanding the taxonomic scope and providing more specific explanations.
Some limitations of our work relate to the availability of dated phylogenies, and the accuracy of the dates therein. Our main source of data, the OneZoom online engine (Rosindell & Harmon, 2012) provides dates that are in agreement with dedicated studies in the case of tetrapod vertebrates (with the exception of some squamate groups), but often disagrees with such studies in the case of seed plants (cf. Magallón et al., 2015). The use of a single age cut-off in our study (the end of the Eocene), combined with the fact that the maps are based on present-day distributions, means that some patterns relevant to this discussion are either not included, or obscured by recent extinctions or range expansion events. A few lineages that are not presently endemic to southern Africa, including East African species, are likely to have survived the end of the Eocene strictly here and colonised northwards only subsequently (e.g. Cordylus; Stanley et al., 2011). Similar expansions are documented in slightly more recent lineages too (Protea; Valente et al., 2010). The diversity of ancient lineages in high-altitude areas may have also changed substantially along the last 30 Myr, with some adapting to colder conditions and colonising uphill (e.g. Guthriea among the Acharieae), while many others, failing to do so, may have secondarily become restricted to the warmer coastal belt. These changes would have been related to global cooling, particularly relevant in high altitudes (e.g. Drakensberg and other eastern mountains), where patches of temperate climate appeared.
The Eocene–Oligocene transition, used here as a cut-off date, is an interesting time in the evolution of plant and animal lineages in Africa and globally, for at least two reasons: climatic cooling, and fire history. Climate-wise, this period was characterised by the abrupt shift to glacial conditions (~34 Myr – permanent glaciation over Antarctica) (Scher & Martin, 2006). Punctuating this, however, there were also periods of warming, which created unstable and overall harsh environmental conditions (Zachos et al., 2001; Tsubamoto et al., 2004). As relevant to fire history, Bond (2015) suggests that fire activity during the Eocene–Oligocene was at an all-time low. Fire frequency only picked up substantially at around 7 Myr (Bond, 2015), and subsequently remained high to the present day, meaning that fire-intolerant lineages that would have been dominant throughout the southern African region 30 Mya have either had to become fire-adapted, or are now restricted to fire-free refugia, more likely to occur along the humid coast or in the rocky landscapes along the Great Escarpment.
Indeed this would match the patterns observed in this study. The total endemism and CWEA maps (Fig. 2) show the coastal regions and the adjacent Great Escarpment as having the highest concentration of ancient endemic lineages. The Great Escarpment is a key geomorphologic feature in southern Africa (Clark, Barker & Mucina, 2011). Mountain ranges provide the environmental heterogeneity likely responsible for the existence of refugia for ancient lineages – not only fire-free, but also climatic (López-Pujol et al., 2011).
Of course, it is worth questioning how the patterns observed here for ancient lineages compare with general species diversity patterns. Several studies have also highlighted the coast and Escarpment as important for species richness and endemism in different taxonomic groups (frogs – Minter et al., 2004; birds – de Klerk et al., 2002; butterflies – Mecenero, Ball & Edge, 2013; plants – Linder, 2014; all vertebrates and selected groups of invertebrates including beetles, weevils, velvet worms and land snails – Perera, 2013). However, in virtually all of these groups there are also species-rich areas further inland, particularly in the north, although endemism is not particularly high there. Furthermore, the patterns presented here, to some extent, coincide with the currently recognised biodiversity hotspots in southern Africa, the CFR, MPA and SK biodiversity hotspots, which are endemism based (Fig. 2). The concentration of ancient endemic lineages in these biodiversity hotspots is of relevance for conservation, as these are major target areas in conservation efforts. In the face of global change, the relative climatic and tectonic stability is proposed as a mechanism in the development of this region as a refugium for ancient lineages, whether these were endemic here to start with, or faced extinction elsewhere (Dynesius & Jansson, 2000; Cowling, Procheş & Patridge, 2009; Habel et al., 2013; Lawson, 2013; Altwegg et al., 2014; Tolley et al., 2014; Bond, 2015).
When considering sister lineage comparisons, our results are also relevant to interpreting broader global distributions. For lineages with widespread distributions, a centre of origin can be suggested by examining their sister lineages (following the ‘out-of’ hypothesis; see Procheş & Ramdhani, 2013). Two lineages were noted to emphasise the ‘out of southern Africa’ scenario. These are the geophyte genera Tulbaghia and Agapanthus, both their sister lineages (core Allioideae and core Amaryllidaceae, respectively; Table S2, Supporting Information) being widespread. Furthermore, the broader Tulbaghia + core Allioideae is sister to Agapanthus + core Amaryllidaceae, indicative of a twofold colonisation of the world out of southern Africa in this broader clade.
Interesting differences in life forms and habitat types between sister lineages and endemic lineages were also noted. Greyia, a family of grassland trees and shrubs, which is sister to Francoaceae (Chilean herbaceous forest dwellers) is one of these examples. Welwitschia, sister to Gnetum, is an even more interesting example – Welwitschia is restricted to the arid Kaokoveld Centre, as opposed to Gnetum which is fairly widespread globally in rainforests. Welwitschia is classified here as an ‘other’ due to its high distinctive growth form, whereas Gnetum range from trees to lianas (climbers) (Table S2, Supporting Information). The irid subfamily Nivenioideae (shrubs) which is sister to Crocoideae (geophytes – as are most other Iridaceae) is another example of growth form divergence, with the shrubby growth form having in this case almost certainly evolved in the Cape fynbos. Several such examples could represent cases of ancient transoceanic vicariance events. The Achariaeae (Achariaceae), sister to Chiangiodendron, are more likely a case of dispersal to southern Africa. Regardless of occupying similar habitats as their sister lineage and other Achariaceae (Table S2, Supporting Information), the Achariaeae are herbaceous or vines, while Chiangiodendron is a tree, as are most other genera in the family whether in southern Africa or elsewhere. Among animals, southern African endemics such as the sugarbirds (Promerops) have remarkable distinctive traits such as longer tail-feathers and bills as opposed to their sister lineage, the East African Modulatrix, a fairly non-descript passerine. The differences between ancient endemic lineages of southern Africa and their sister lineages, further justify that these lineages are uniquely contributing to global trait diversity.
Conclusion
This study paints a remarkable picture of multiple, morphologically distinct plant and animal lineages having survived regionally since Eocene days. It makes a novel contribution towards the incorporation of phylogenetics in biodiversity mapping, as it not only accounts for the evolutionary history of species (as phylogenetic diversity does) but also emphasises the importance of old, monotypic lineages (cf. Purvis et al., 2000; Isaac et al., 2007; Rosauer et al., 2009; Tucker & Cadotte, 2013). Taxon-based conservation approaches can be enhanced by focusing particularly on distinctive species, such as ancient ones (Isaac et al., 2007), or even by accounting for higher taxa or evolutionary lineages. The evolutionary history of lineages is important, as this determines evolutionary distinctiveness of species (Sechrest et al., 2002; Lamoreux et al., 2006). This, in turn, ensures that feature diversity is preserved (Forest et al., 2007). The ancient endemic lineages selected in this study are good examples of preserving feature diversity, as many of these differ substantially from their closest relatives. Should these lineages go extinct; the unique features of these lineages will be lost (Sechrest et al., 2002; Lamoreux et al., 2006; Forest et al., 2007). Additionally, the delineation of southern African (and global) hotspots still remains inadequate (Perera et al., 2011). It has also been pointed out that the ‘hotspot approach’ to conservation hinders public participation in conservation efforts by removing the focus on species (Purvis et al., 2000; Isaac, Mallet & Mace, 2004; Isaac et al., 2007).
We argue that ancient lineages can serve a twofold purpose in conservation efforts. First, ancient lineages can be used as ‘flagship lineages’ in the prioritisation of new conservation areas (Fig. 2A, B), where this may still be necessary, even though southern Africa can be viewed as a pioneer in terms of conservation planning (Cowling et al., 1999, 2003; Brooks et al., 2006; Simth et al., 2008). Flagship lineages can, in this context, promote public participation in conservation efforts. Second, ancient lineages can also be used in assessing the functioning of current conservation areas towards preserving these distinctive plants and animals (cf. Tucker & Cadotte, 2013).
Of course, the method delineated here can be applied to lineages of any given age. A global approach to this effect is possible for much older lineages, with no endemism criterion added (Procheş et al., 2015), while more recent lineages could even be mapped across a much finer scale. We consider this method to be an intuitive way of mapping lineage distributions using the age cut-off as an objective criterion, unlike taxonomic diversity, which is based on arbitrarily-defined taxonomic ranks. At the same time, the method is more focused and interpretable than phylogenetic diversity mapping, which compressing multiple patterns in a single measure.
Acknowledgements
John Measey, Sandun Perera and two anonymous reviewers are thanked for comments on an earlier version, and Aaron Bauer for providing unpublished data for the genus Afroedura. This study is based on the MSc dissertation of ALP (supported by DAAD-NRF funding). ŞP also acknowledges NRF funding for rated researchers.




