Morphological scaling of body form in four shark species differing in ecology and life history
Abstract
Body form can change across ontogeny, and can influence how animals of different sizes move and feed. Scaling data on live apex predatory sharks are rare and, therefore, we examined patterns of scaling in ontogenetic series of four sympatric shark species exhibiting a range of sizes, ecologies and life histories (tiger, bull, blacktip, and nurse shark). We evaluated 13 linear morphological variables and two areas (caudal and dorsal) that could influence both animal condition and locomotor performance. These measurements included dimensions of the dorsal, pectoral, and caudal fins, as well as several dimensions of body circumference, and of the head. For all four species, the body axis (eye-to-eye, lateral span, frontal span, proximal span) scaled close to isometry (expected slope of 1.0). The two largest sharks (tiger and bull sharks) also showed significant negative allometry for elements of the caudal fin. We found significant negative allometry in the lengths of the upper lobe of the caudal fin (caudal fin 1) and the overall height of the caudal fin (caudal fin 2) in tiger and bull sharks, with slopes ranging from about 0.60 to 0.73. Further, tiger sharks showed negative allometry in caudal fin area. These results suggest that in terms of overall body dimensions, small sharks are roughly geometrically similar to large sharks, at least within the species we examined. However, juvenile tiger (and to a lesser extent bull sharks) are notable in having proportionately larger caudal fins compared to adult sharks. As the caudal fin contributes to generating thrust during forward locomotion, this scaling implies differences among adult and juvenile sharks in locomotor ability. © 2014 The Linnean Society of London, Biological Journal of the Linnean Society, 2014, 114, 126–135.
Introduction
How morphological shape changes with size, or the study of allometry, has been of significant interest in the broader field of evolutionary biology and functional morphology for over 100 years (Hill, 1950; Calder, 1984; Schmidt-Nielsen, 1984; LaBarbera, 1989; Brown & West, 2000). The study of scaling informs us about the broader process of adaptation by understanding how animals change their ecological, physiological, and behavioral strategies as they grow larger, either during ontogeny, or among species at a macroevolutionary level (Emerson, 1978; LaBarbera, 1989; Carrier, 1996; Brown & West, 2000; Herrel & O'Reilly, 2006) For example, the scaling of metabolic rate to body size among vertebrates has shed light on how animals of different sizes invest in different energetic strategies, which consequently affects their mode of feeding, reproduction, and many other traits (Calder, 1984; Schmidt-Nielsen, 1984; O'Reilly, Lindstedt & Nishikawa, 1993; Birch, 1999; Tyler-Bonner & Horn, 2000). Other studies have shown how deviations from isometry can shed light on how species have evolved. Fox example, the highly positive allometry of bite forces among and within lizard species (Meyers, Herrel & Birch, 2002) suggests strong selection for higher bite forces as lizard become larger. This pattern likely occurs because destructive biting is most effective as a fighting strategy in large lizards, which bite harder than small lizards.
The allometry of body shape and function has been examined in a number of fish species (e.g. Eggold & Motta, 1992; Richard & Wainwright, 1995; Cook, 1996; Lauder et al., 2003; Danos & Lauder, 2007; Habegger et al., 2011; Reiss & Bonnan, 2010), including some shark species (Ferry-Graham, 1998; Wilga & Lauder, 2002, 2004a, b; Lingham-Soliar, 2005; Huber, Weggelaar & Motta, 2006). However, there are few data on the allometry of body and fin shape in larger predatory shark species, especially for live specimens, with some exceptions (Lingham-Soliar, 2005). Gathering data on live specimens is important, especially for larger sharks, due to practical and ethical issues. First, the volume of the body can change dramatically upon preservation (Shields & Carlson, 1996) and populations of many sharks are experiencing rapid global declines (Worm et al., 2013), such that non-lethal methods are needed to study these species (Hammerschlag & Sulikowski, 2011). Both tiger sharks (Galeocerdo cuvier) and bull sharks (Carcharhinus leucas) are apex predators, and therefore, how these predators change in body shape and overall function as they mature may provide insight into the differential movement patterns of adults and juveniles, especially given that both species move long distances (Hammerschlag et al., 2012a, b). Modeling studies have examined possible energetic costs of migration in sharks (Smith & Caldwell, 2010), but these studies have focused on average adult body forms, and have not considered possible shape changes across ontogeny.
We examined allometric changes in 13 linear morphological variables and two areas (caudal fin areas and dorsal fin areas) in live individuals of four shark species: tiger, bull, blacktip (Carcharhinus limbatus), and nurse (Ginglymostoma cirratum). We chose morphological variables that could influence both locomotion and the ability to feed, which included dimensions of the dorsal, pectoral, and caudal fins, as well as several dimensions of body circumference, and of the head. We focused on these shark species for two reasons. First, all co-occur sympatrically in coastal areas of the Caribbean. Second, these species show a range of ecologies and lifestyles. Tiger and bull sharks are apex predators in tropical seas, reaching large sizes (above 300 cm) that can consume large, diverse, prey (e.g. other sharks, large fish, turtles, birds, marine mammals) (Cortes, 1999; Compagno, Dando & Fowler, 2005). Both species also travel large distances (1000 s of km, e.g. Hammerschlag et al., 2012a, b). By contrast, blacktip sharks are significantly smaller (maximum length less than 300 cm), and occupy significantly smaller home ranges compared to tiger and bull sharks (Heupel, Simpfendorfer & Hueter, 2004). Further, blacktip sharks typically consume fish species and are themselves potential prey for both tiger and bull sharks (Cortes, 1999; Heupel et al., 2004; Wetherbee, Cortes & Bizzaro, 2004). Finally, nurse sharks are medium-sized species (maximum length less than 300 cm), with relatively circumscribed home ranges (Heist et al., 2003), and which consume a range of crustaceans and smaller fishes (Castro, 2000). This range of species allowed us to explore for potential species, size and trophic guild differences in morphological scaling. We asked the following questions: (1) How does morphology scale within species for tiger, bull, blacktip, and nurse sharks? (2) How do these scaling coefficients differ among these species? and (3) Can differences in scaling coefficients among species be explained by differences in their size and trophic guild?
Material and Methods
Capturing sharks
Sharks were captured using standardized circle-hook drumlines following Hammerschlag et al. (2012b) and Gallagher et al. (2014). Briefly, drumlines were composed of a weighted base that sits on the seafloor. Attached to the weight was a 23-m monofilament line (400 kg test) that terminated in a baited 16/0 offset circle hook. The gear was left for 1 h before retrieval. When a shark was captured, it was restrained in the water alongside the back of the boat or secured to a partially submerged platform. With the exception of a nurse shark, which can ventilate its gills through buccal pumping, a hose was then placed in the mouths' of tigers, bulls and blacktip sharks (ram ventilators) to pump fresh seawater over its gills to enable the shark to breathe. Sharks were sampled at various locations in Southern Florida and the Northern Bahamas between July 2012, and December 2013. We captured a total of 185 sharks (Fig. 1; tiger shark: N = 45, bull shark: N = 29, blacktip shark: N = 47, nurse shark: N = 64) (Table 1). We were able to gather good size ranges (approximately 2x to 3x variation in PCL pre-caudal length (PCL)) for each of these species (Table 1).

Images of the four study species: Top left: Tiger shark. Top right: Bull shark. Bottom left: Blacktip shark. Bottom right: Nurse shark. Image of tiger shark by Neil Hammerschlag; images of bull, blacktip and nurse shark by Christine Shepard.
| Tiger shark | Bull shark | Blacktip shark | Nurse shark | |
|---|---|---|---|---|
| Pre-caudal length | 207.9 ± 8.3 (107.0–303.0) | 161.7 ± 4.3 (130.0–196.0) | 110.5 ± 2.7 (62.0–141.0) | 159.7 ± 2.5 (115.0–195.0) |
| Eye-to-Eye | 31.7 ± 1.21 (16–46) | 29.7 ± 0.9 (23.0–40.5) | 15.6 ± 0.3 (10.0–19.5) | 23.6 ± 0.4 (13.0–29.2) |
| Lateral span | 69.5 ± 2.8 (36–108) | 65.6 ± 2.1 (46.0–89/0) | 42.2 ± 1.1 (24.0–58.0) | 54.7 ± 1.1 (29.0–71.5) |
| Frontal span | 72.10 ± 3.30 (34.5–111.0) | 71.1 ± 2.6 (52.0–104.0) | 45.5 ± 1,2 (27.0–64.5) | 56.7 ± 1.5 (31.0–81.0) |
| Dorsal span | 66.3 ± 3.1 (31.0–111.0) | 59.2 ± 2.1 (45.0–91.5) | 39.8 ± 1.1 (21.0–56.5) | 35.9 ± 0.9 (19.0–58.5) |
| Caudal keel Circ. | 30.51 ± 1.1 (15.0–46.0) | 27.8 ± 0.7 (22.0–35.5) | 19.2 ± 0.5 (11.0–27.0) | 26.7 ± 0.5 (15.0–36.0) |
| Pectoral fin | 34.9 ± 1.6 (14.0–53.0) | 39.0 ± 1.0 (31.0–51.0) | 23.2 ± 0.6 (12.0–28.5) | 34.1 ± 0.8 (19.0–61.0) |
| Dorsal fin 1 | 30.2 ± 1.2 (15.0–44.0) | 32.8 ± 0.9 (23.5–40.5) | 21.2 ± 0.5 (11.5–27.0) | 28.6 ± 0.5 (17.0–35.5) |
| Dorsal fin 2 | 22.7 ± 0.8 (10.0–33.0) | 25.7 ± 0.5 (21.0–31.0) | 18.0 ± 0.5 (10.0–25.0) | 22.6 ± 0.4 (13.0–30.5) |
| Dorsal fin 3 | 25.8 ± 0.9 (14.0–37.0) | 27.4 ± 1.1 (19.5–41.0) | 17.3 ± 0.5 (10.0–24.0) | 21.5 ± 0.6 (9.0–38.0) |
| Caudal fin 1 | 67.4 ± 1.8 (40.0–90.0) | 57.6 ± 1.2 (47.5–68.5) | 40.0 ± 0.9 (23.0–49.0) | 61.1 ± 1.2 (37.0–77.5) |
| Caudal fin 2 | 70.6 ± 2.1 (42.0–96.0) | 60.0 ± 1.2 (49.5–71.0) | 42.2 ± 1.0 (22.0–54.5) | 60.3 ± 1.1 (33.0–74.0) |
| Caudal fin 3 | 30.1 ± 1.1 (16.0–43.0) | 26.5 ± 0.7 (19.0–33.0) | 17.1 ± 0.5 (7.5–24.0) | 16.8 ± 0.9 (7.5–68.5) |
Morphological measurements
- (1)
Head size (EE; distance between eyes, from the inner part of the eyes);
- (2)
Lateral span (LS): the distance spanning (i.e. around the curved dorsal ‘back’ of the shark) from the insertion point of the anterior edge of one pectoral fin to the same point on the other pectoral fin;
- (3)
Frontal span (FS): the distance spanning (i.e. around the curved dorsal ‘back’ of the shark) from the insertion point of the anterior edge of the dorsal fin to a line oriented parallel to the horizontal plane of the pectoral fin;
- (4)
Proximal span (PS): the distance spanning (i.e. around the curved dorsal ‘back’ of the shark) from the insertion point of the posterior edge of the dorsal fin to a line oriented parallel to the horizontal plane of the pectoral fin;
- (5)
Caudal keel circumference (CKC; total circumference at the base of the tail as measured at the caudal keel);
- (6)
Pectoral fin length (PFL; the linear distance from the insertion of the pectoral fin at the distal edge to the tip of the pectoral fin when fully extended);
- (7)
Dorsal fin 1 (DF1; Distance from the anterior insertion point of the dorsal fin to the tip of the dorsal fin);
- (8)
Dorsal fin 2 (DF2; Distance from the tip of the dorsal fin to the posterior insertion point of the dorsal fin);
- (9)
Dorsal fin 3 (DF3; distance horizontally across the sharks body between the anterior and posterior insertion points of the dorsal fin);
- (10)
Caudal fin 1 (CF1; the linear distance from the insertion of the caudal fin to the tip of the caudal fin);
- (11)
Caudal fin 2 (CF2; the linear distance from the tip of the caudal fin to the ventral tip of the bottom part of the caudal fin);
- (12)
Caudal fin 3 (CF3; the linear distance from the bottom anterior edge of the caudal fin to the bottom posterior edge of the caudal fin; and
- (13)
Pre-caudal length (PCL; the linear distance from the tip of the snout to the pre-caudal pit).
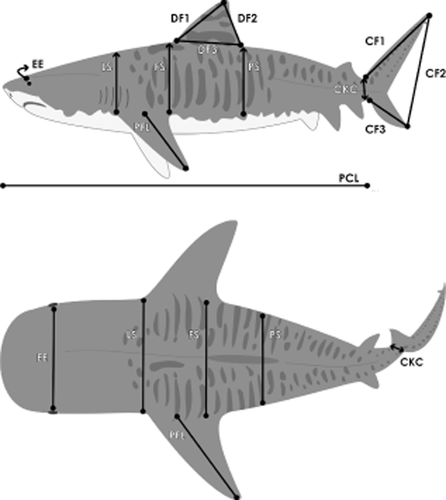
A diagram of a tiger shark with the morphological variables measured in this study depicted. Note that all four variables shown (LS = lateral span, FS = frontal span, PS = proximal span, and CKC = caudal keel circumference) along the body axis of the shark were all measured across the body (see Materials and methods above for more exact landmarks of morphological measurements).
We also estimated areas of the dorsal fin (Dorsal fin area) and caudal fin (Caudal fin area) using Heron's formula in which area = sqrt(p*(p – a)*(p – b)*(p – c)) where p is the perimeter of the triangle and a, b, and c are each side of the triangle.
Statistical analyses
We calculated scaling slopes by linear least-square regression plotting total PCL as the independent metric of size on the x-axis, and the above shape variables (EE, FS, LS, PS, PFL, CKC, DF1, DF2, DF3, CF1, CF2, CF3, Caudal fin area, Dorsal fin area) as the dependent variables on the y-axis. All variables were ln-transformed prior to scaling analyses. We tested the predictions that each of the shape variables scales isometrically through standard t-tests comparing each slope versus the expected value of 1.0 (linear measures) or 2.0 (area measures). We performed each of these analyses within each of the four shark species. We used P < 0.05 as our metric of statistical significance. We did not employ multiple comparison corrections (e.g. Bonferroni test), as these metrics tend to be overly conservative (Perneger, 1998). As in any statistical analysis, we provide the magnitude of P-values to allow the reader to assess the biological realism of the results for themselves.
Results
All of the morphological variables for all of the species showed strong and positive relationships with body length (PCL, Figs 3, 4). The r-squared values between body length and the morphological variables we examined ranged from 0.31 to 0.91, with the majority of relationships showing values greater than 0.75. In general, all five body axis variables (eye-to-eye, lateral span, frontal span, proximal span, and caudal keel circumference) had scaling coefficients that did not differ significantly from the expected isometric slope of 1.0 (Table 2). The notable exception to this trend was a significant pattern of negative allometry in the eye-to-eye measure in blacktip sharks (slope = 0.78). The pattern of isometric growth also largely held for the pectoral fins for all four species (Table 2), which had slopes ranging from 0.87 (bull sharks) to 1.07 (nurse sharks), none of which differed significantly from the isometric slope of 1.0. The three dorsal fin measures showed a range of scaling coefficients. For example, dorsal fin 1 (dorsal fin height) showed a range of slopes from 0.62 (nurse shark) to 0.91 (blacktip shark) (Table 2). The lone significant deviation from isometry in the dorsal fin was dorsal fin 2 in bull sharks (slope = 0.71). In contrast to the body axis measures, the three caudal fin measures showed consistent statistically significant deviation from isometry. The two largest sharks (tiger and bull sharks) each showed significant negative allometry in caudal fin 1 (length of upper lobe of caudal fin) and caudal fin 2 (total height of caudal fin, Table 2). In general, nurse sharks were the exception, with slopes more closely approaching 1.0 for two of the three caudal fin measures (Table 2). The dorsal and caudal fin areas showed significant negative allometry in tiger sharks (expected slope = 2, Table 3), and were not significantly different from isometry in the other species.
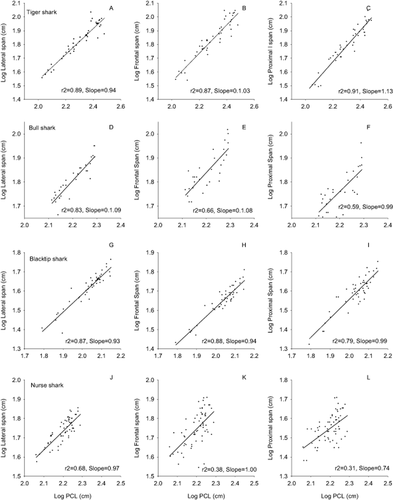
Scatterplots for four shark species between log-transformed values of PCL (x-axis) and three body axis measurements (Lateral span, Frontal span, Proximal span, y-axis, all log-transformed).
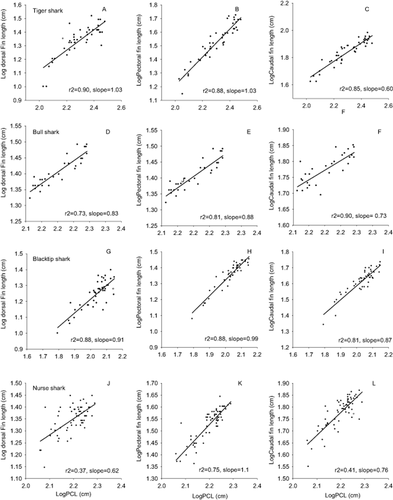
Scatterplots for four shark species between log-transformed values of PCL (x-axis) and three fin measurements (Pectoral fin length, Dorsal fin height, Caudal fin height, y-axis, all log-transformed).
| Tiger shark | Bull shark | Blacktip shark | Nurse shark | |
|---|---|---|---|---|
| Eye-to-Eye | 0.92 ± 0.04 | 0.96 ± 0.10 | 0.79 ± 0.05* | 1.00 ± 0.06 |
| Lateral span | 0.94 ± 0.05 | 1.09 ± 0.10 | 0.93 ± 0.05 | 0.97 ± 0.08 |
| Frontal span | 1.03 ± 0.06 | 1.09 ± 0.15 | 0.94 ± 0.05 | 1.00 ± 0.16 |
| Dorsal span | 1.13 ± 0.05 | 0.99 ± 0.16 | 0.99 ± 0.07 | 0.74 ± 0.14 |
| Caudal keel Circ. | 0.85 ± 0.04 | 0.78 ± 0.09 | 0.89 ± 0.06 | 0.91 ± 0.09 |
| Pectoral fin | 1.03 ± 0.06 | 0.87 ± 0.08 | 0.99 ± 0.05 | 1.07 ± 0.08 |
| Dorsal fin 1 | 0.88 ± 0.05 | 0.83 ± 0.10 | 0.91 ± 0.05 | 0.62 ± 0.10 |
| Dorsal fin 2 | 0.75 ± 0.08 | 0.71 ± 0.06* | 0.86 ± 0.09 | 0.68 ± 0.10 |
| Dorsal fin 3 | 0.68 ± 0.08 | 1.13 ± 0.17 | 0.85 ± 0.11 | 1.00 ± 0.20 |
| Caudal fin 1 | 0.60 ± 0.04*** | 0.73 ± 0.05* | 0.84 ± 0.04 | 0.76 ± 0.11 |
| Caudal fin 2 | 0.67 ± 0.04** | 0.61 ± 0.09* | 0.87 ± 0.06 | 0.96 ± 0.09 |
| Caudal fin 3 | 0.87 ± 0.05 | 0.70 ± 0.14 | 0.85 ± 0.11 | 1.03 ± 0.18 |
- *P < 0.05, **P < 0.01, ***P < 0.005.
| Tiger shark | Bull shark | Blacktip shark | Nurse shark | |
|---|---|---|---|---|
| Dorsal fin area (means) | 287.1 ± 17.8 | 344.6 ± 19.9 | 150.4 ± 7.5 | 242.3 ± 9.2 |
| Caudal fin area (means) | 1031.6 ± 62.8 | 748.6 ± 33.5 | 343.0 ± 16.0 | 471.2 ± 21.4 |
| Dorsal fin area (slopes) | 1.58 ± 0.09* | 1.79 ± 0.17 | 1.73 ± 0.14 | 1.72 ± 0.22 |
| Caudal fin area (slopes) | 1.50 ± 0.07* | 1.40 ± 0.16 | 1.76 ± 0.11 | 1.75 ± 0.35 |
- *P < 0.05, **P < 0.01, ***P < 0.005.
Discussion
Our analysis of the scaling of 13 linear morphological variables and two fin areas in four shark species showed several patterns for different parts of the body. In general, for all four species examined, four of five body axis variables (eye-to-eye, lateral span, frontal span, proximal span) all scaled close to isometry for most of the species (expected slope of 1.0). The lone exception to this trend was a pattern of significant negative allometry in head size (EE) in blacktip sharks (discussed further, see below). In tiger and bull sharks, elements of the dorsal fin displayed some negative allometry, notably dorsal fin area in tiger sharks. The strongest scaling pattern among the variables examined was significant negative allometry in the lengths of the upper lope of the caudal fin (caudal fin 1), the overall height of the caudal fin (caudal fin 2) and caudal fin area in tiger sharks. Bull sharks also exhibited negative allometry in the above caudal fin linear measures, but caudal fin area did not deviate significantly from isometry in this species.
Taken together, these results suggest that in terms of overall body dimensions, small sharks are roughly geometrically similar to large sharks, at least within the species we examined. However, for tiger (and to a lesser extent bull) sharks, there is a clear pattern of relatively smaller caudal fins in adults compared to juveniles. As these features may contribute to locomotion in sharks, our data suggest the potential for ontogenetic differences in locomotion as well, a point that obviously requires investigation.
Departures from allometric expectations typically arise either because of basic constraints on how body proportions can change with size (e.g. growth of the human head), or because of differing selective pressures on animals of differing sizes (Hill, 1950; Calder, 1984; Schmidt-Nielsen, 1984; LaBarbera, 1989; Brown & West, 2000). The primary question here is how our result of negative allometry for the tiger shark caudal fin fits into this framework. In another analysis of the caudal fin dimensions of white sharks Carcharodon carcharias, Lingham-Soliar (2005) provided a similar result in showing negative allometry of the area of the caudal fin in this large shark. Further, bull sharks also showed significant negative allometry for some linear measures of the caudal fin, but caudal fin area did not show a significant deviation from isometry. This latter result suggests that there may be additional interesting aspects of ontogenetic shape change in bull sharks that would worthy of more detailed investigation. By contrast, in a thorough analysis of a small shark species (the spiny dogfish, Squalus acanthias), Reiss & Bonnan (2010) found isometric scaling of the caudal fin using geometric morphometrics. Thus, scaling of the caudal fin appears to differ among shark species of different sizes.
As the caudal fin is the primary agent of propulsion in sharks (Wilga & Lauder, 2002, 2004a, 2004b; Lauder et al., 2003; Flammang et al., 2011), differences among juveniles and adult sharks in the relative proportions of the caudal fin may impact their locomotion in nature. Shark species differ in the overall shape of the caudal fin, which shows a close match with locomotor style (Wilga & Lauder, 2002, 2004a, b; Flammang et al., 2011). For example, fast-moving pelagic sharks such as white sharks and mako sharks (Isurus) exhibit more symmetric tails that in combination with other features, enable rapid bursts of high-speed locomotion. By contrast, slower-moving sharks such as nurse sharks tend to have more asymmetric tails in which the upper lobe is oscillated slowly to enable slower cruising. Deviation from isometry can often arise within species as a result of increased predation pressure on juveniles (Carrier, 1996). Given that adult tiger and bull sharks will consume small sharks, it is plausible that the relatively larger caudal fins in juveniles of these species reflects the commitment to increased locomotor effort for younger sharks to avoid predation. Although not mutually exclusive, smaller fins may allow these species to catch faster moving smaller prey that are not consumed as adults. In order to test this idea more fully, it would be necessary to examine locomotion in the field, such as through examining accelerometers and satellite tags (Hammerschlag, Gallagher & Lazarre, 2011). Given the close match between fin shape and lifestyle among shark species, the ontogenetic variation observed here could also influence locomotion in adult and juvenile tiger sharks, but more anatomical and morphometric data are needed before any firm conclusions can be drawn.
Head shape in sharks is related both to diet and to locomotor style (e.g. Huber et al., 2006; Lowry, Motta & Heuter, 2007). For example, the broad and blunt heads of tiger and bull sharks are likely related in part to their habit of grasping and consuming large prey such as turtles and smaller sharks (Cortes, 1999). However, head shape can also impact drag profile. Given that there are also ontogenetic changes in lifestyle in sharks, understanding the impacts of the scaling of the head is also valuable. Among the species we examined here, head dimensions scaled isometrically with the exception of blacktip sharks, which showed negative allometry of the head, resulting in larger blacktip sharks with relatively narrower heads (as measured at the eye) compared to juvenile blacktip sharks. A narrower head is usually associated with diminished drag in sharks. Interestingly, in an analysis of the scaling of bite force in blacktip sharks, Huber et al. (2006) showed that bite force seems to scale with positive allometry, likely because of an increase in mechanical advantage as these sharks mature. Similarly, leopard sharks (Triakis semifasciata) also show negative allometry in the head, yet increased musculature, resulting in a narrower but likely stronger profile as they grow larger (Lowry et al., 2007). Therefore, there may be competing demands for rapid locomotion (due to a narrower head), yet relatively increased bite capacity, as blacktip sharks mature. Additional data on blacktip locomotion of different sizes, as well as more data on the prey hardness, might shed light on these patterns.
Finally, we note that our results could impact how researchers model long-distance movements in larger sharks. The negative scaling of caudal fin dimensions, along with the isometric growth of the body axis in tiger and bull sharks found in our study could impact the energetics of movement, especially for movements across long distances. While some valuable studies have modeled long-distance movement in blue sharks (Prionace glauca, Smith & Caldwell, 2010), these studies did not consider how ontogenetic changes in morphology might influence movement energetics. For example, future models such as used by Smith & Caldwell (2010) could examine accurate morphological replicas of adults and juveniles which incorporates changes in allometry. Field and laboratory observations of movement in adult and juvenile sharks could then inform whether sharks modify the kinematics of motion as they mature. Incorporation of more realistic morphological models and kinematic data into energetic models of migration might reveal constraints on migration tactics of sharks across a range of body sizes.
Acknowledgements
For their invaluable help with morphological data collection, we are very grateful to the students, staff and interns of University of Miami's RJ Dunlap Marine Conservation Program (RJD). We especially thank Kyra Hartog and Daniela Escontrela for helping initiate the field sampling and assisting with much data collection. For their invaluable help with data entry, we thank Catherine Macdonald, Lindsay Jennings and Christian Pankow. Several anonymous reviewers provided helpful comments on a prior version of this paper. Funding support was provided in part by RJD, the Batchelor Foundation, and the Disney Worldwide Conservation Fund. Research was carried out under the University of Miami Animal Care and Use Protocol 12-280 under research permits from Florida Keys National Marine Sanctuary, Florida Fish and Wildlife Conservation Commission, NOAA National Marine Fisheries Service, Everglades National Park, Biscayne National Park and Bahamas.




