Are black flies of the subgenus Wilhelmia (Diptera: Simuliidae) multiple species or a single geographical generalist? Insights from the macrogenome
Abstract
Organisms with vast distributions often represent geographical mosaics of cryptic species. The black fly Simulium (Wilhelmia) lineatum is among the most widely distributed members of the family Simuliidae, ranging from the British Isles to eastern China. Rather than viewing S. lineatum as a possible aggregate of multiple species, taxonomists have suggested a more inclusive taxon with additional synonyms. Accordingly, S. lineatum is an ideal candidate for testing the hypothesis that a wide geographical distribution signals the presence of more than one species. A cytogenetic approach was used to probe the macrogenome of S. lineatum and other taxa proposed by taxonomists as conspecific. The banding patterns in the polytene chromosomes of 480 larvae from 15 countries across the Palearctic Region revealed 128 rearrangements of the complement. All rearrangements were autosomal and 89% were inversions nonrandomly distributed among species and among chromosome arms. The analyses clarify long-standing confusion over previously proposed names and reveal a longitudinal succession of four species sequentially replacing one another from west to east: Simulium lineatum s.s., Simulium balcanicum, Simulium turgaicum, and Simulium takahasii. Thus, S. turgaicum is recalled from synonymy and the other three species are validated. Within the most-represented species, S. balcanicum, the frequency of inversions follows a longitudinal gradient with a north–south bias; as the distance between the sites increases along this north-west–south-east axis, the similarity of inversion frequencies between sites decreases. Validation of the concept that broadly distributed black flies are composites of structurally similar species provides a framework for guiding discovery of additional biodiversity. © 2014 The Linnean Society of London, Biological Journal of the Linnean Society, 2014, 114, 163–183.
Introduction
Much of ecology and applied biology is focused at the species level: from understanding the effects of specialization on metacommunity dynamics (Pandit, Kolasa & Cottenie, 2009) to controlling the vectors of disease agents and managing pests (Adler, 2009; Kamali et al., 2012). Species perceived as generalists, however, often involve cases of mistaken identity; putative species with broad host or geographical ranges tend to be composites of structurally similar (cryptic) species, each occupying a discrete subset of the larger range (Williams et al., 2012; Hambäck et al., 2013).
The family Simuliidae, through study of the giant polytene chromosomes in the larval silk glands, provides classic examples of cryptic biodiversity in taxa once considered single widespread species occupying diverse habitats (Rothfels, 1979; Adler, Cheke & Post, 2010). Current examples of generalists in the Simuliidae are found in the Palearctic Simulium subgenus Wilhelmia. Among the 26 species in the subgenus are some of the most widely distributed black flies in the Old World (Adler & Crosskey, 2014). Simulium lineatum (Meigen) as currently recognized, for example, extends from southern Spain (Gallardo-Mayenco & Toja, 2002) and the British Isles across a vast swath of the Palearctic through the Altai south to Iran and eastward to the coast of China, a distance of more than 9500 km (Adler & Crosskey, 2014). Although wide distributions typically signal the presence of cryptic species (Adler et al., 2004, 2010), S. lineatum has been viewed as broadly cohesive, and two former species, Simulium salopiense Edwards of Western Europe and Simulium turgaicum Rubtsov of Central Asia, were declared conspecific with S. lineatum (Crosskey & Davies, 1972; Crosskey & Howard, 1997). Populations of Wilhelmia in Japan and Korea were considered conspecific with S. lineatum until 1962, when they were described under the name Simulium takahasii (Rubtsov), although this was based on questionable structural differences. More recently, Simulium balcanicum (Enderlein), which differs structurally only in the pupal stage by having a petiolate pair of gill filaments, was posited as conspecific with S. lineatum (Crosskey & Zwick, 2007).
The chromosomes of seven nominal species of the subgenus Wilhelmia have been described (Grinchuk & Chubareva, 1974; Knoz, Grinchuk & Chubareva, 1976; Petrova et al., 2003; Chubareva, Petrova & Reva, 2007; Chubareva & Petrova, 2008; Huang et al., 2012). Only one study, however, attempted a banding comparison between species [Simulium equinum (L.) and S. lineatum in south-western Germany (Weber & Grunewald, 1989)] and only one study, although limited to Armenia, considered the possibility of cryptic species (in Simulium paraequinum Puri) (Petrova et al., 2003). All chromosomally known members of the subgenus Wilhelmia share a whole-arm interchange (IS + IIL, IL + IIS) possibly homologous with that in the African subgenus Metomphalus (Vajime & Dunbar in Rothfels, 1979). The only other examples of a IS + IIL, IL + IIS interchange in the Simuliidae (Rothfels & Freeman, 1983; Procunier, 1982) are of independent origin in distantly-related taxa (i.e. different genera).
Our initial motivation for a cytogenetic study of Wilhelmia was sparked by a severe outbreak of black flies that affected tourism and livestock production along the Kizilirmak River in Turkey's Cappadocia Region during the middle of the first decade of 2000 (Yilmaz et al., 2007; Sariözkan et al., 2014). To determine the species involved in the Cappadocia outbreak, we conducted a cytogenetic analysis of the population of Wilhelmia in the Kizilirmak River. To reconcile the outbreak with the pest status of the relevant species in other regions and to place our results in an interpretive context, we adopted a collaborative approach and expanded the geographical coverage to include the entire Palearctic Region. Our primary objective was to test the hypothesis that S. lineatum is a single species, as some taxonomists have claimed, versus a composite of one or more of the variously recognized species or synonyms: S. balcanicum, S. lineatum s.s., S. salopiense, S. takahasii, and S. turgaicum. We examined material from populations typically regarded as representative of each of these names.
Material and Methods
Collection of material
Larvae were hand collected from trailing vegetation at 18 sites in 15 countries (Table 1, Fig. 1) and fixed in two or three changes of modified Carnoy's solution (1 : 3 acetic ethanol). Larvae patently infected with parasites (morphologically identified) were recorded (Tables 2, 3). Larval carcasses and photographic negatives of chromosomes were deposited in the Clemson University Arthropod Collection, Clemson, SC.
| Site | Location | Latitude, longitude | Elevation (m a.s.l.) | Date | Species |
|---|---|---|---|---|---|
| 1 | Armenia, Kotayk Province, Hrazdan, Hrazdan River, below reservoir | 40°30′31″N 44°44′23″E | 1695 | 9 June 1999 | Simulium turgaicum |
| 2 | Austria, Stopfenreuth, side channel of Danube River |
48°08′43″N 16°53′26″E |
140 | 8 May 2011 | Simulium balcanicum |
| 3 | France, Corsica, Calviani, Tavignano River |
42°07′02″N 09°29′26″E |
6 | 17 September 2013 | Simulium lineatum |
| 4 | Germany, Ruhstorf, Rott River | 48°25′44″N 13°20′01″E | 315 | 3 March 2012 | Simulium balcanicum, Simulium lineatum |
| 5 | Germany, near Landshut, Isar River | 48°30′31″N 12°04′23″E | 401 | 11 March 2012 | Simulium lineatum |
| 6 | Greece, Aliakmonas, Aliakmonas (= Haliacmon) River |
40°18′10″N 21°25′59″E |
550 | 3 May 2013 | Simulium balcanicum |
| 7 | Iran, Isfahan, Nazhvan, Zayandehrood River |
32°38′26″N 51°37′58″E |
1580 | 26 March 2011 | Simulium turgaicum |
| 8 | Italy, San Paolo, Tagliamento River |
45°53′16″N 12°56′21″E |
15 | 22 September 2013 | Simulium lineatum |
| 9 | Japan, Oita Prefecture, Yufu, Yufuin, tributary of Oita River | 33°15′51″N 131°22′02″E | 465 | 10 December 2013 | Simulium takahasii |
| 10 | Macedonia, Demir Kapija, Vardar River |
41°24′24″N 22°16′14″E |
100 | 2 May 2013 | Simulium balcanicum |
| 11 | Poland, Stary Sacz, Dunajec River |
49°34′57″N 20°37′58″E |
290 | 14 June 2013 | Simulium lineatum |
| 12 | Romania, Simeria Veche, Strei River |
45°50′09″N 23°02′48″E |
190 | 17 September 2012 | Simulium balcanicum |
| 13 | Romania, Bârza, Cerna River |
44°50′26″N 22°23′15″E |
110 | 25 September 2011 | Simulium balcanicum |
| 14 | Romania, Băile Herculane, Cerna River |
44°54′05″N 22°25′47″E |
180 | 12 April 2012 | Simulium balcanicum |
| 15 | Serbia, Velika Plana, Velika Morava River |
44°20′26″N 21°07′25″E |
90 | 1 May 2013 | Simulium balcanicum |
| 16 | Slovakia, Mužla, Danube River |
47°46′05″N 18°32′19″E |
102 | 13 May 2010 | Simulium balcanicum |
| 17 | Turkey, Kizilirmak River* | 38°N 34°E | 925 | 2010–2012 | Simulium balcanicum, Simulium turgaicum |
| 18 | United Kingdom (England), East Stoke, River Frome | 50°40′56″N 02°10′59″W | 7 | 6 April 2013 | Simulium lineatum (as Simulium salopiense) |
- *Given the proximity of collections (within approximately 20 km of one another), small samples (S. balcanicum), and minimal polymorphisms (S. turgaicum), chromosomal data are combined in Tables 2 and 3 for 4 sites on the Kizilirmak River: Avanos (38°42′57.30″N 34°50′27.50″E), 925 m, 18 October 2010; Gülşehir Bridge, 38°45′22.26″N 34°37′01.15″E, 905 m, 13 October 2011; Gülşehir-Hacibaektaş Road, 38°45′38.30″N 34°36′39.29″E, 904 m, 13 October 2011; and Gülşehir, 38°45′25.17″N 34°36′55.32″E, 904 m, 24 September 2011 and 15 January 2012.
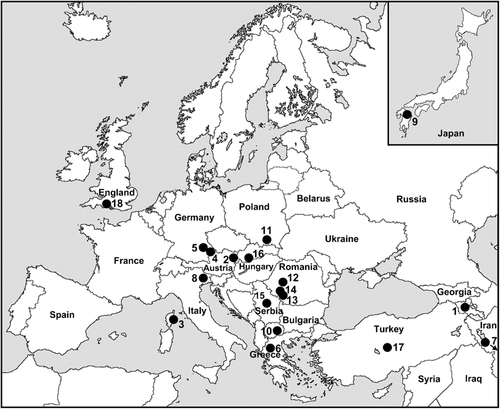
Map of collecting sites for four species of Simulium (Wilhelmia) in the Palearctic Region; numbers correspond to sites in Table 1.
| Site | 2 | 4a | 6 | 10a | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|
| Females : Males | 6 : 1 | 21 : 7 | 1 : 0 | 18 : 15 | 18 : 10 | 5 : 2 | 0 : 1 | 22 : 12 | 7 : 2 | 10 : 7 |
| IS-2 | 0.03 | |||||||||
| IS-4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IS-8 | 0.02 | |||||||||
| IS-9 | 0.06 | |||||||||
| IS-18 | 0.02 | |||||||||
| IL-1 | 0.79 | 0.93 | 0.47b | 0.61c | 0.43 | 0.49b | 0.89 | 0.62 | ||
| IL-11 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IL-13 | 0.29 | 0.16 | 0.12 | 0.23b | 0.07 | 0.07 | 0.33 | |||
| IL-14 | 1.00 | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 |
| IL-15 | 0.04 | |||||||||
| IL-16 | 0.06 | |||||||||
| IIS-1 | 0.29 | 0.27 | 0.50 | 0.39b | 0.34 | 0.29 | 0.50 | 0.32b | 0.33 | 0.68 |
| IIS-2d | 0.03 | |||||||||
| IIS-3d | 0.02 | 0.03 | ||||||||
| IIS-4 | 0.03 | |||||||||
| IIS-5 | 0.02 | 0.03 | ||||||||
| IIS-6 | 0.14 | 0.04 | 0.02 | 0.09 | 0.06 | 0.06 | 0.09 | |||
| IIS-7 | 0.03 | |||||||||
| IIS-8 | 0.03 | |||||||||
| IIS-9d | 0.03 | |||||||||
| IIS-10d | 0.03 | |||||||||
| IIS-11 | 0.03 | |||||||||
| IIS-12 | 0.03 | |||||||||
| IIS-13 | 0.03 | |||||||||
| IIS-15 | 0.04 | 0.07 | ||||||||
| IIS-16 | 0.06 | 0.04 | 0.03 | |||||||
| IIS-17 | 0.04 | 0.03 | 0.02 | 0.14 | 0.01 | 0.06 | ||||
| IIS-18 | 0.02 | |||||||||
| IIS-19 | 0.03 | |||||||||
| IIS-20d | 0.02 | 0.02 | 0.50 | |||||||
| IIS-21 | 0.02 | |||||||||
| IIS-22 | 0.03 | |||||||||
| IIS-23 | 0.02 | |||||||||
| IIS-24 | 0.50 | 0.05 | 0.04 | |||||||
| IIS-26d | 0.02 | |||||||||
| IIS-27 | 0.02 | |||||||||
| IIS-28 | 0.01 | |||||||||
| IIS-29 | 0.04 | 0.03 | ||||||||
| IIS-30 | 0.04 | 0.03 | ||||||||
| IIS-31d | 0.01 | |||||||||
| IIS-32 | 0.02 | |||||||||
| IIS-complexde | 0.02 | 0.06 | 0.06 | |||||||
| IIS 54hb | 0.07 | |||||||||
| IIL-1 | 0.79 | 0.95 | 1.00 | 0.82 | 0.96 | 0.93 | 1.00 | 0.94 | 0.78 | 1.00 |
| IIL-2 | 0.32 | |||||||||
| IIL-4 | 0.29 | |||||||||
| IIL-6 | 0.03 | |||||||||
| IIL-7 | 0.07 | 0.04 | 0.05 | 0.06 | 0.32 | |||||
| IIL-8 | 0.17 | 0.04 | 0.07 | 0.04 | ||||||
| IIL-11 | 0.07 | 0.02 | 0.06 | 0.04 | 0.03 | 0.17 | ||||
| IIL-12 | 0.07 | 0.02 | 0.09 | 0.04 | 0.03 | |||||
| IIIL-1 | 0.07 | 0.13 | 0.02 | 0.07 | 0.04 | 0.06 | 0.03 | |||
| IIIL-7 | 0.02 | 0.06 | ||||||||
| IIIL-8 | 0.07 | 0.05 | ||||||||
| IIIL-9 | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IIIL-10 | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| IIIL-11 | 0.02 | |||||||||
| IIIL-12 | 0.11 | 0.07 | ||||||||
| IIIL-13 | 0.06 | |||||||||
| IIIL-15 | 0.04 | 0.01 | ||||||||
| IIIL 97hb | 0.07 | |||||||||
| IIIL 100hb1 | 0.02 | |||||||||
| Mean no. heterozygous autosomal inversions/larva | 2.29 | 1.02 | 2.00 | 2.42 | 2.39 | 2.14 | 2.00 | 0.99 | 3.00 | 3.77 |
- a Site 4 : one male larva was infected with the microsporidium Polydispyrenia simulii. Site 10 : one female larva was infected with a mermithid nematode.
- b The following inversions were in Hardy–Weinberg equilibrium (d.f. = 1, P > 0.05): IL-1 at Site 10 (ss = 10, si = 15, ii = 8, where s = standard and i = inverted; χ2 = 0.25) and Site 15 (ss = 11, si = 13, ii = 10; χ2 = 1.87); IL-13 at Site 12 (ss = 18, si = 7, ii = 3; χ2 = 2.50); IIS-1 at Site 10 (ss = 11, si = 18, ii = 4; χ2 = 0.67) and Site 15 (ss = 15, si = 16, ii = 3; χ2 = 0.19).
- c IL-1 was not in Hardy–Weinberg equilibrium (ss = 7, si = 8, ii = 13; χ2 = 4.50, d.f. = 1, P < 0.05).
- d The inversion occurred on top of the IIS-1 sequence.
- e The breakpoints of overlapping heterozygous inversions on top of a IIS-1 homozygote were not resolved in three larvae; the unresolved inversions at the 3 different sites (12, 16, and 17) were not necessarily the same inversion.
| Site | Simulium lineatum | Simulium takahasii | Simulium turgaicum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5a | 8 | 11 | 18a | 9 | 1 | 7a | 17a | |
| Females : Males | 5 : 7 | 0 : 1 | 9 : 10 | 4 : 2 | 1 : 0 | 31 : 18 | 19 : 30 | 1 : 0 | 22 : 12 | 82 : 61 |
| IS-1 | 0.01 | |||||||||
| IS-3 | 0.03 | |||||||||
| IS-4 | 1.00 | 0.76 | 1.00 | 0.50 | 1.00 | |||||
| IS-5 | 0.05 | 0.06 | ||||||||
| IS-6 | 1.00 | 0.32 | 1.00 | 0.50 | 0.16b | |||||
| IS-7 | 0.03 | 0.08 | ||||||||
| IS-10 | 0.04 | |||||||||
| IS-11 | 0.02 | |||||||||
| IS-12c | 0.07 | |||||||||
| IS-13c | 0.07 | |||||||||
| IS-14c | 0.07 | |||||||||
| IS-15c | 0.07 | |||||||||
| IS-16c | 0.07 | |||||||||
| IS-17 | 0.01 | |||||||||
| IS N.O.d | 0.01 | |||||||||
| IL-2 | < 0.01 | |||||||||
| IL-3ae | < 0.01 | |||||||||
| IL-3be | 0.32b | |||||||||
| IL-4 | < 0.01 | |||||||||
| IL-5 | < 0.01 | |||||||||
| IL-6 | < 0.01 | |||||||||
| IL-7 | < 0.01 | |||||||||
| IL-8 | < 0.01 | |||||||||
| IL-9 | < 0.01 | |||||||||
| IL-10 | 0.88 | 0.84 | 1.00 | 1.00 | ||||||
| IL-11 | 0.96 | 0.55 | 1.00 | 0.98 | ||||||
| IL-12 | 0.03 | |||||||||
| IL-17 | 0.39b | |||||||||
| IL-18 | 0.24b | |||||||||
| IL-19 | 0.50 | |||||||||
| IL-20 | 0.04 | |||||||||
| IL-21 | 0.01 | |||||||||
| IL 29hb | 1.00 | |||||||||
| IL 34hb | 0.13 | |||||||||
| IL 35hb | 0.06 | |||||||||
| IL 41hb | < 0.01 | |||||||||
| IL 29hb | 1.00 | |||||||||
| IIS-14 | 0.08 | 0.42 | ||||||||
| IIS-25 | 0.50 | |||||||||
| IIS 45i | 0.01 | |||||||||
| IIL-3 | 0.05 | |||||||||
| IIL-5 | < 0.01 | |||||||||
| IIL-8 | 0.58 | 1.00 | 0.66b | 1.00 | 0.39b | |||||
| IIL-9 | 1.00 | 0.50b | 1.00 | 0.81 | ||||||
| IIL-10 | 0.21 | 0.08 | ||||||||
| IIL-11 | 0.58 | 0.50 | 0.66 | 0.83 | 1.00 | 0.35b | ||||
| IIL-12 | 0.29 | |||||||||
| IIL-13 | 0.03 | |||||||||
| IIL-14 | 0.16 | 0.08 | 0.08 | |||||||
| IIL-15f | 0.07 | |||||||||
| IIL-16f | 0.07 | |||||||||
| IIL-17 | 0.01 | |||||||||
| IIL-18 | 0.01 | |||||||||
| IIL-19g | 0.02 | |||||||||
| IIL-20 | 0.01 | |||||||||
| IIL-21 | 0.01 | |||||||||
| IIL 63hb | 0.08 | |||||||||
| IIIS-1 | 0.01 | |||||||||
| IIIS-2 | 0.13 | 0.01 | ||||||||
| IIIS-3 | 1.00 | |||||||||
| IIIS-4 | 0.02 | |||||||||
| IIIS 77hb | < 0.01 | |||||||||
| IIIL-2 | < 0.01 | |||||||||
| IIIL-3 | < 0.01 | |||||||||
| IIIL-4 | < 0.01 | |||||||||
| IIIL-5 | 1.00 | 0.50 | 0.96 | 1.00 | 1.00 | 1.00 | ||||
| IIIL-6 | 0.50 | 0.03 | ||||||||
| IIIL-10 | 1.00 | |||||||||
| IIIL-14 | 0.07 | |||||||||
| IIIL-complex | 0.50 | 0.05 | ||||||||
| IIIL 99i | 1.00 | |||||||||
| IIIL 100hb2 | 0.01 | |||||||||
| Mean no. heterozygous autosomal inversions/larva | 1.42 | 5.00 | 4.68 | 1.33 | 4.00 | 4.31 | 0.12 | 0.00 | 0.03 | 0.12 |
- a Site 5 : 1 male larva was infected with the microsporidium Amblyospora sp., and 1 male larva was infected with a mermithid nematode. Site 18: one male larva was infected with Amblyospora varians. Site 7: one male larva was infected with Amblyospora bracteata. Site 17: one male larva was infected with the microsporidium Polydispyrenia simulii; one male larva was infected with Amblyospora bracteata, and breakpoints of a mid-IS heterozygous inversion were not determined for this individual.
- b The following inversions were in Hardy-Weinberg equilibrium (d.f. = 1, P > 0.05): IS-6 at Site 18 (ss = 35, si = 12, ii = 2, where s = standard and i = inverted; χ2 = 0.53); IL-3b at Site 18 (ss = 24, si = 18, ii = 7; χ2 = 1.33); IL-17 at Site 18 (ss = 21, si = 18, ii = 10; χ2 = 2.51); IL-18 at Site 18 (ss = 31, si = 14, ii = 5; χ2 = 2.70); IIL-8 at Site 5 (ss = 4, si = 5, ii = 10; χ2 = 3.28) and Site 18 (ss = 17, si = 26, ii = 6; χ2 = 0.68); IIL-9 at Site 5 (ss = 5, si = 9, ii = 5; χ2 = 0.05); IIL-11 at Site 18 (ss = 21, si = 22, ii = 6; χ2 = 0.00).
- c Five inversions (IS-12, IS-13, IS-14, IS-15, and IS-16) were completely linked and paired with the standard homologue in seven larvae (six females, one male).
- d Heterozygous for nucleolar organizer (N.O.) expression.
- e Although IL-3a and IL-3b have the same macro-breakpoints, they must be independent inversions because IL-3b occurred only if IL-10 was present, whereas IL-3a occurred without IL-10.
- f IIL-15 and IIL-16 were completely linked and were found in all, and only, the same seven larvae with linked inversions IS-12, IS-13, IS-14, IS-15, and IS-16.
- g IIL-19 occurred only on top of IIL-9.
Chromosome preparation
Polytene chromosomes were prepared using the Feulgen technique of Rothfels & Dunbar (1953), in accordance with the procedures outlined by Adler & Huang (2011), although modified by using a cold, 30-min treatment in 5 N hydrochloric acid (Charalambous et al., 1996). The silk-gland contents of larvae typically solidified into a gelatinous mass within as little as a week of fixation; thus, after Feulgen staining, the chromosomes were stripped from the mass with fine needles. Gender was determined by gonadal shape. The ovoid to spherical male gonads of the subgenus Wilhelmia are among the largest in the Simuliidae; their large size and melanin sheath made gender determination straightforward. Female gonads were elongated and ensheathed with melanin only at the distal end.
Chromosome interpretation
Selected chromosomes from larvae collected in Turkey's Kizilirmak River in September and October 2011 and in Japan in December 2013 were photographed under oil immersion on an Olympus BX40 compound microscope. Maps (Figs 2, 3, 4, 5, 6, 7, 8, 9, 10) were prepared using PHOTOSHOP ELEMENTS, version 8 (Adobe Inc.). The entire complement (chromosomes I, II, and III) was divided into 100 arbitrary sections of approximately equivalent length, beginning with the short arm (S) of I and continuing through the long arm (L) of III. We maintained the section limits of Weber & Grunewald (1989) wherever possible, although our attempts were stymied by the low resolution of their maps and the presence of heterozygous inversions (e.g. in the middle of IS) and geographically local, homozygous inversions (e.g. in the base of IIL) on their maps. For example, the IL, IIL, and IIIL photomaps for S. lineatum of Weber & Grunewald (1989) carry the IL-10, 11 and IIL-8, 9, 14, and IIIL-5 homozygous sequences, respectively, of our system, and IS in their photomap is heterozygous for our IS-4 sequence. We therefore established the standard map de novo, based on the most common sequence in each arm across our sampled populations. Chromosomal rearrangements, chiefly inversions and heterobands, are indicated by brackets or arrows on our maps. Inversions typically were numbered in order of their discovery. Each heteroband (hb) was named for the section in which it occurred (e.g. IIIL 97hb), as were supernumerary bands (e.g. IIIL 99i). Fixed inversions across all sites of a species are italicized.
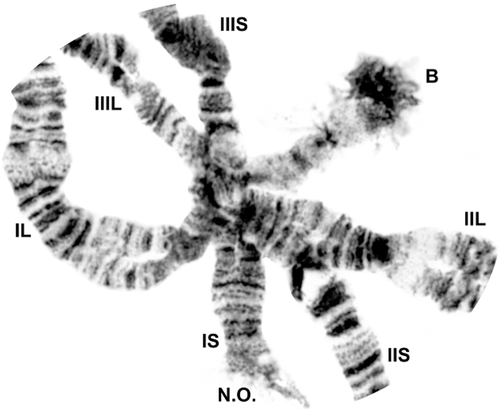
Chromocentre of male larva of Simulium turgaicum, showing bases of six chromosome arms (IS–IIIL), B chromosome (B), and nucleolar organizer (N.O.)
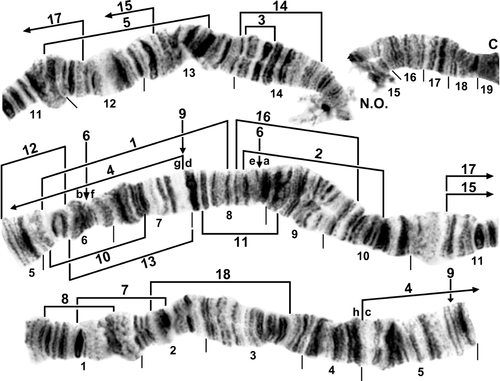
Standard sequence for IS arm of female larva of Simulium turgaicum. C, centromere; N.O., nucleolar organizer. Limits of inversions IS-1–IS-18 of various species are indicated by arrows or brackets. Ordering fragments indicated by the letters ‘a’ through ‘h’ will produce the IS-4, 6 sequence in Simulium lineatum.
Standard sequence for IL arm of female larva of Simulium turgaicum. C, centromere; hb, location of heteroband; m, marker (sensu Rothfels et al., 1978). Limits of inversions IL-2–IL-21 of various species are indicated by brackets. The limits of inversion IL-14 of Simulium balcanicum are bracketed.
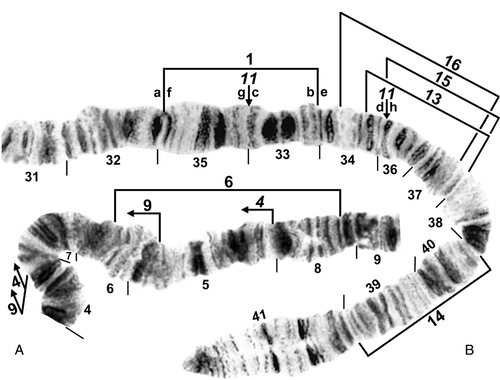
Chromosome I of female larva of Simulium balcanicum. A, IS (sections 4–9 only). The IS-4 sequence is present, with limits bracketed. Limits of inversions IS-6 and IS-9 are indicated by brackets. B, IL (sections 31–41 only). IL-1, IL-11, and IL-14 are present, with inversion limits indicated by brackets (IL-1, IL-14) or arrows (IL-11). Ordering fragments indicated by the letters ‘a’ through ‘h’ will produce the standard sequence. Limits of inversions IL-13, IL-15, and IL-16 are indicated by brackets.
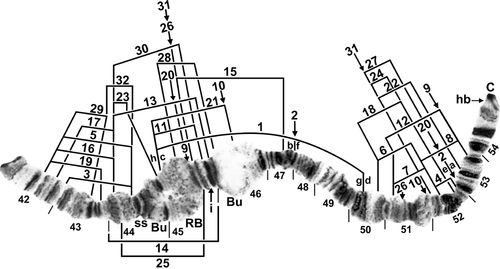
Standard sequence for IIS arm of female larva of Simulium turgaicum. Limits of inversions IIS-1–IIS-32 are indicated by arrows or brackets. Inversions above the chromosome are specific to Simulium balcanicum; those below the chromosome are specific to Simulium lineatum. Inversions IIS-2, IIS-3, IIS-9, IIS-10, IIS-20, IIS-26, and IIS-31 occur only on top of the IIS-1 inversion. Bu, bulge; C, centromere; hb, location of heteroband; i, location of band insert; RB, ring of Balbiani; ss, shoestring marker; the two halves of the bulge are separated by the ring of Balbiani. Ordering fragments indicated by the letters ‘a’ through ‘h’ will produce the IIS-1, 2 sequence of S. balcanicum.
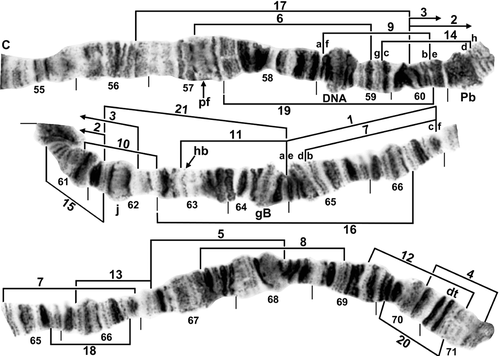
Standard sequence for IIL arm of female larva of Simulium turgaicum. C, centromere; DNA, DNA puff; dt, doublet; gB, grey band; hb, location of heteroband; j, jagged marker; Pb, parabalbiani; pf, puffing band. Limits of inversions IIL-1–IIL-21 of various species are indicated by brackets. Ordering fragments indicated by the letters ‘a’ through ‘h’ will produce the IIL-9, 14 sequence of Simulium lineatum, and ordering the letters ‘a’ through ‘f’ will produce the IIL-1, 7 sequence of Simulium balcanicum.
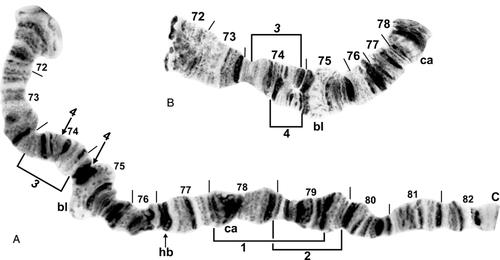
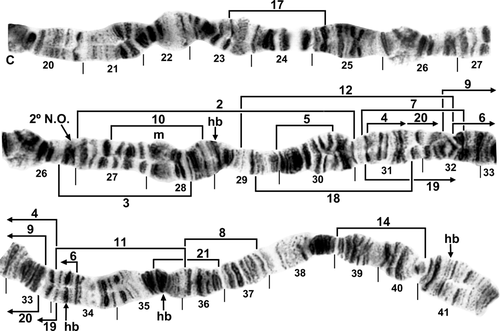
Chromosome IIIS of Simulium (Wilhelmia) species. bl, blister; C, centromere; ca, capsule; hb, heteroband (shown as heterozygous). A, standard sequence for IIIS arm of female larva of Simulium turgaicum, showing suppressed terminal flaring (sections 72–73). Limits of inversions IIIS-1 and IIIS-2 and fixed inversion IIIS-3 are indicated by brackets; autosomal inversion IIIS-4 of Simulium takahasii, indicated by arrows, occurs only on top of IIIS-3; the two inversions share near-coincident proximal breakpoints such that IIIS-4 is based on the fixed IIIS-3 sequence. B, male larva of S. takahasii; IIIS-3 and IIIS-4 as a heterozygote (inverted on lower homologue) are present.
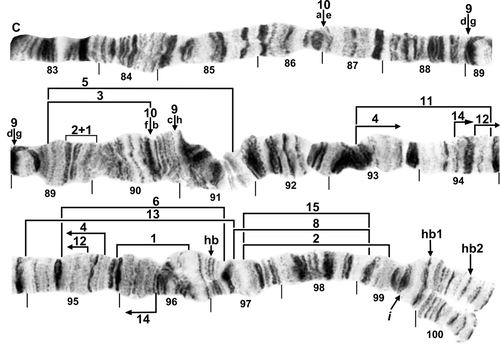
Standard sequence for IIIL arm of female larva of Simulium turgaicum. C, centromere; hb, location of heteroband; 2 + 1, marker with two black bands and one grey band, possibly homologous with bands in section 93 of the standard sequence for the subgenus Simulium. Limits of inversions IIIL-1–IIIL-6, IIIL-8, and IIIL-11–IIIL-15 are indicated by brackets. Positions of heterobands in section 97 of Simulium balcanicum and one each in section 100 of S. balcanicum (hb1) and Simulium lineatum (hb2), and of a band insert in section 99 of Simulium takahasii (i) are indicated by arrows. Ordering fragments indicated by the letters ‘a’ through ‘h’ will produce the IIIL-9, 10 sequence of S. balcanicum.

Chromosome IIIL of female larvae of Simulium (Wilhelmia) species. A, Simulium balcanicum (sections 83–92 only) with the typical IIIL-9, 10 sequence; arrows indicate inversion breakpoints. Letters ‘a’ through ‘h’ indicate the sequence of chromosome fragments necessary to produce the standard IIIL sequence from the IIIL-9, 10 sequence. C = centromere, 1 + 2 = marker with one grey band and two black bands. Limits of inversion IIIL-7 are indicated by bracket. B, Simulium turgaicum, showing the standard sequence (sections 89–94 only); arrow in section 92 indicates sticky heterochromatin; 2 + 1 indicates the same marker as in section 89 of Fig. 9A but with reversed polarity. C, Simulium takahasii [sections 83–91(half) and 98–100 only] with fixed inversion IIIL-10 and characteristic band insert in section 99.
Frequencies of all polymorphisms, based on individual chromosomal homologues, were calculated (Tables 2, 3). Twenty-four (4.8%) of 504 chromosomally prepared larvae could not be compared completely with the standard map and were excluded from calculations of polymorphism frequencies and all other evaluations. Fifteen of these larvae were from Europe's late-summer generation (September) of minuscule mature larvae (length, mean ± SE = 5.3 ± 0.07 mm, N = 14) of S. lineatum s.s. The remaining nine individuals (all S. balcanicum) with chromosomes not amenable for analysis were pharate pupae. Polymorphisms of sufficient frequency were tested for Hardy–Weinberg equilibrium. We used chi-squared tests to evaluate independence of inversion sequences and gender, and goodness of fit tests to determine if the distribution of inversions was equal among species and among chromosome arms.
Gill morphology
The lengths of all eight gill filaments (Fig. 11) were measured using IMAGEJ (NIH) for representative pupae of S. balcanicum from Site 2 (N = 2); S. lineatum from Sites 3 (N = 7), 8 (N = 3), and 18 (N = 1); S. takahasii from Beijing, China (N = 3); and S. turgaicum from Sites 7 (N = 3) and 17 (N = 5). Principal components analysis was used to display differences in gill measurements including the ratios of filaments 5 : 2 and 3 : 1 in multidimensional space.
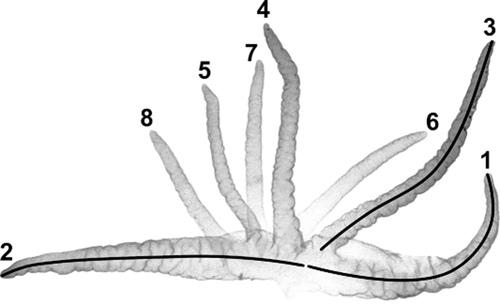
Pupal gill of Simulium lineatum from Corsica, France, showing numbering system for the eight filaments and examples of measurements (lines) for filaments 1, 2, and 3.
Statistical analysis of inversion frequencies
Only S. balcanicum was collected in adequate numbers (7–34 larvae/site) over a sufficient number of sites (N = 8) for analysis of inversion frequencies. Samples were collected across north–south and east–west gradients (Table 1). These gradients represent not only changes in location, but also measures of distance. Correlations were therefore used to compare the relationships of distance to degrees north and east. These correlations were based on all pairwise comparisons of sites, rendering the data inherently non-independent; significance tests therefore were based on 1000 random permutations (Lunneborg, 2000). Regression analysis was used to determine whether the distance gradients between sites had directionality. For example, are sites in more northern locations also found in western, as opposed to eastern, locations? Because there is no predictor or response variable in this case, the regression equation was computed using reduced major-axis regression (Sokal & Rohlf, 1995), with degrees north arbitrarily assigned to the left side of the equation. A distance–decay relationship (DDR) between site similarity, based on inversion-frequency profiles, and Euclidean distance between sites was examined using linear regression (Soininen, McDonald & Hillebrand, 2007). Similarity between sites was calculated as the Bray–Curtis resemblance coefficient of the square-root transformed frequency of inversions (McCune & Grace, 2002). We established a priori that the frequency of an inversion had to be greater than zero for at least two of the eight sites to be included in the analysis. Significance tests of the DDR were based on 1000 random permutations (Lunneborg, 2000). Both similarity and distance were expressed on a log-log scale for this regression. By definition, when a site is compared to itself, it will have 100% similarity in the inversion profile and zero distance, which could increase the overall strength of the DDR unfairly. Accordingly, we used a trimmed data set in which these values were removed. Relationships between individual inversions and degrees north and east were examined with simple linear regression (log-log scale).
Results
Generalities
The characteristic Wilhelmia whole-arm interchange (IS + IIL, IL + IIS) was present in all larvae. The centromeres of the three metacentric chromosomes were associated tightly in a persistent chromocentre in all nuclei. Although additional heterochromatin that would suggest a genuine chromocentre (Fig. 2) was not evident in the majority of populations, it was present, although minimal, in some populations (e.g. Site 10). Ectopic pairing of telomeres occurred in some populations of S. balcanicum (e.g. Site 15) and S. lineatum (e.g. Sites 3 and 11). The telomeres, especially of IIS and, to a lesser extent IIIS (Fig. 8B), of S. takahasii typically had extra heterochromatin. Sticky heterochromatin in IIIL, manifesting as bands adhering to one another, particularly in section 92 (Fig. 10B), was typical of populations at some sites (e.g. S. balcanicum at Site 10, S. turgaicum at Site 17, and, less frequently, S. takahasii at Site 9).
Classic chromosomal markers that are widespread or universal in the Simuliidae (Rothfels, Feraday & Kaneps, 1978), such as the parabalbiani, were present in all material (Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10). The doublet marker (dt; Fig. 7), a conspicuous subterminal pair of bands in IIL (section 71), although taxonomically more restricted, minimally to the subgenus Wilhelmia, provided utility as an additional marker. The nucleolar organizer was in the base of IS (Figs 2, 3). The bulge in IIS was cleaved by the ring of Balbiani (Fig. 6). The end of IIIS was typically flared, obscuring the banding in section 72. Clusters of chromatids could be discerned in the flared region at some sites (e.g. Site 17), producing a braided appearance. Flaring of the IIIS terminus was suppressed in some nuclei of S. balcanicum (e.g. Sites 10, 15, and 17), S. turgaicum (Site 17; Fig. 8A), and S. takahasii (Site 9).
More than 110 inversions were found among 480 larvae, with 59 in S. balcanicum (N = 165 larvae), 41 in S. lineatum (N = 88 larvae), six in S. takahasii (N = 49), and 14 in S. turgaicum (N = 178). No rearrangements were significantly linked (P > 0.05) to gender in any population. The sex chromosomes, therefore, were microscopically undifferentiated (X0Y0) in all populations. Additional rearrangements, particularly heterobands, were distributed among the four species (Tables 2, 3).
Simulium balcanicum
Ten sites had populations of S. balcanicum (N = 165). Two inversions, IS-4 and IL-11 (Fig. 5), were fixed in all individuals of S. balcanicum, and three additional inversions (IL-14, IIIL-9, and IIIL-10) (Figs 4, 10A) were almost fixed (> 0.98), with only two larvae heterozygous for IL-14 and one heterozygous for IIIL-9 and IIIL-10. Three additional inversions had high representation in S. balcanicum across sites. IL-1 (Fig. 5B) was found in 61.8% of all homologues, and IIS-1 (Fig. 6) in 39.7%. The latter inversion served as the base sequence on top of which at least eight additional inversions were built (Table 2). IIL-1 (Fig. 7) occurred in 91.2% of all homologues across sites, and was fixed in Turkish samples. Inversion IIL-7 (Fig. 7), which occurred only on top of IIL-1, reversed the majority of the IIL-1 inversion back into the standard orientation. Approximately 50 additional polymorphic inversions and three rare heterobands (Figs 6, 9) were found across the sampled range of S. balcanicum (Table 2). Five of six inversions with representation sufficient for analysis in three populations (Sites 10, 12, and 15) were in Hardy–Weinberg equilibrium (Table 2).
Mean heterozygosity varied from 1.0 in Germany to approximately 3.8 in Turkey, with one larva in Turkey having seven heterozygous inversions. Thirteen polymorphisms were shared by half or more of the 10 populations. Within the hyperactive IIS arm, inversion breakpoints were clustered in three hotspots (sections 42–44, 45–46, and 50–53; Fig. 6).
Collection sites of S. balcanicum showed significant (P < 0.01) correlations between distance and degrees north (r = 0.942) and degrees east (r = 0.962). Regression analysis indicated that distance between sites followed a north-west to south-east axis; thus, sites in northern locations were found west of sites in more southern locations (Fig. 12). The DDR analysis showed a significant (P < 0.01) negative relation between similarity of inversion profiles of S. balcanicum and distance; as the distance between sites increased along a north-west to south-east axis, the similarity of inversion frequencies between sites decreased (Fig. 13). Of the four inversions analyzed individually, two (IL-13 and IIS-1) showed significant (P < 0.05) log-linear relationships with direction (Table 4).
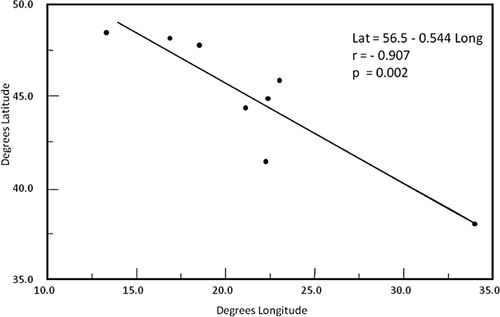
Reduced major-axis regression between latitude and longitude coordinates for eight sites from which chromosomal inversion profiles of Simulium balcanicum were analyzed.

Distance–decay relationship between site similarity, based on chromosomal inversion profiles of Simulium balcanicum, and distance between sites.
| Inversion | Gradient | Slope | r2(adj) | F1,6 | P |
|---|---|---|---|---|---|
| IL-1 | Latitude | 3.67 | 0.104 | ||
| IL-1 | Longitude | 2.35 | 0.176 | ||
| IL-13 | Latitude | 0.0247 | 52.6% | 8.76 | 0.025 |
| IL-13 | Longitude | 3.74 | 0.101 | ||
| IIS-1 | Latitude | –0.0321 | 73.1% | 20.00 | 0.004 |
| IIS-1 | Longitude | 0.0198 | 78.6% | 26.76 | 0.002 |
| IIL-1 | Latitude | 1.25 | 0.306 | ||
| IIL-1 | Longitude | 1.80 | 0.228 |
Simulium lineatum
The six European populations of S. lineatum (N = 88) were united by high frequencies (> 0.15) of shared inversions (IS-4, IS-6, IL-10, IL-11, IIL-8, IIL-9, IIL-11, and IIIL-5). Of 41 total inversions, 28 were found in England (N = 49 larvae), 21 in Germany (N = 19), 11 in Italy (N = 6), nine in France (Corsica) (N = 12), and eight in the single larva from Poland. The mean number of heterozygous inversions per larva ranged from 1.3 in Italy to more than 4.3 in England and Germany, with one larva in Germany having eight heterozygous inversions and two in England having 13 each. Of the 11 inversions common to England and Germany, four [all in high frequency (> 0.50) in Germany] were fixed (IS-4, IL-10, and IIIL-5) or almost fixed (0.98 for IL-11) in England. With five exceptions (IL-3b, IL-17, IL-18, IIL-10, and IIL-12), all inversions unique to either England or Germany were in low (< 0.09) frequency. All inversions for which genotypic frequencies were adequate for statistical analysis (Site 5: IIL-8 and IIL-9; Site 18: IS-6, IL-3b, IL-17, IL-18, IIL-8, and IIL-11) were in Hardy–Weinberg equilibrium (Table 3). Six heterobands were found (Figs 4, 7, 9; Table 3).
The English population demonstrated complete inversion linkage within chromosomes; the inverted sequences for IS-12, IS-13, IS-14, IS-15, and IS-16 (Fig. 3) were traced on the same homologue (i.e. in cis conformation) in all seven larvae (six females, one male). These same seven larvae also showed 100% linkage (cis conformation) of IIL-15 and IIL-16 (Fig. 7). None of these IS or IIL inversions appeared independently. We cannot be certain, however, that the IS and IIL inversions came from the same parent. The complement of these seven individuals was no more loosely paired than that of typical individuals.
The following eight inversions in our study of S. lineatum were equivalent to those (in parentheses) found by Weber & Grunewald (1989): IS-4 (= IS-4), IS-5 (= IS-6), IIS-14 (= IIS-5), IIL-8 (= IIL-7), IIL-9 (= IIL-3), IIL-11 (= IIL-5), IIL-12 (= IIL-4), and IIL-14 (= IIL-8).
Simulium takahasii
One sample of S. takahasii (N = 49) from Japan was available for study (Table 1). All 49 larvae had the same banding sequence, with two fixed inversions, IIIS-3 (Fig. 8B) and IIIL-10 (Fig. 10C). A darkly staining, glassy band of variable thickness was homozygously inserted in section 99 of all larvae; the chromosome typically was constricted at the band (Fig. 10C). Polymorphisms were minimal (frequencies < 0.02), with only four floating inversions, heterozygous expression of the nucleolar organizer, and a heterozygous band insert in section 45 of IIS (Fig. 6, Table 3). The level of inversion heterozygosity in the population, consequently, was low (0.12).
Simulium turgaicum
The three West Asian populations of S. turgaicum (N = 178) were characterized by a low level of polymorphism. Of 14 total inversions, 13 were found in central Turkey (N = 143 larvae) and one in Iran (N = 34), all with frequencies of 0.01 or less (Table 3). IIIS-1 in our system differed by one band from IIIS-1 of Weber & Grunewald (1989). The mean number of heterozygous inversions per larva was less than 0.15 per site. Large supernumerary (B) chromosomes were present in one female and two male larvae from Turkey. They paired at one end with the chromocentre in approximately 40% of the nuclei, suggesting a near-terminal centromere (Fig. 2). The puffed distal region possibly signalled the presence of a nucleolar organizer (Procunier, 1975). Two heterobands in low frequency (< 0.01) were discovered (Figs 4, 8; Table 3).
Chromosomal comparisons
The distribution of inversions differed significantly among the four species (χ2 = 47.06, d.f. = 4, P < 0.001) (Table 5). Simulium balcanicum differed from all other species by two fixed inversions (IS-4 and IL-11), two almost fixed (i.e. frequencies ≥ 0.98) inversions (IL-14 and IIIL-9), and a unique set of polymorphisms. It shared IIIL-10 with S. takahasii, which had one unique fixed inversion (IIIS-3). No rearrangements were shared between S. balcanicum and S. turgaicum. However, five inversions were shared between S. balcanicum and S. lineatum: IS-4, IL-11, IIL-8, IIL-11, and IIL-12. The number of inversions across chromosome arms was not independent of the two species (χ2 = 29.894, d.f. = 4, P < 0.001; IIIS was omitted from analysis because of only one inversion) (Table 5). Simulium lineatum, S. takahasii, and S. turgaicum shared no rearrangements. Simulium lineatum could be distinguished from S. turgaicum by its high frequency of inversions (e.g. IS-4, IL-10, IL-11, IIL-9, and IIIL-5), which typically were present in half or more of the chromosomal homologues at each site. No hybrids were detected at any sites where S. balcanicum occurred in the same rivers with S. lineatum (Germany) and S. turgaicum (Turkey).
| Larvae (N) | Inversions (N) | IS | IL | IIS | IIL | IIIS | IIIL | |
|---|---|---|---|---|---|---|---|---|
| Simulium balcanicum | 165 | 59 | 5 | 6 | 31 | 8 | 0 | 9 |
| Simulium lineatum | 88 | 41 | 13 | 8 | 2 | 13 | 1 | 4 |
| Simulium takahasii | 49 | 6 | 0 | 1 | 0 | 2 | 2 | 1 |
| Simulium turgaicum | 178 | 14 | 1 | 8 | 0 | 1 | 1 | 3 |
Morphological comparisons
Only the gill of S. balcanicum, with its petiolate pair of filaments, was consistently distinguishable from the gills of the other three species. A principal components analysis of gill lengths and selected ratios suggested that S. takahasii was distinct but that S. turgaicum was indistinguishable from S. lineatum, although sample sizes were small (Fig. 14). The first two axes of the principal components analysis explained 83.7% of the total variation in gill morphology; therefore, only these two were used to graph gill dimensions in multidimensional space.
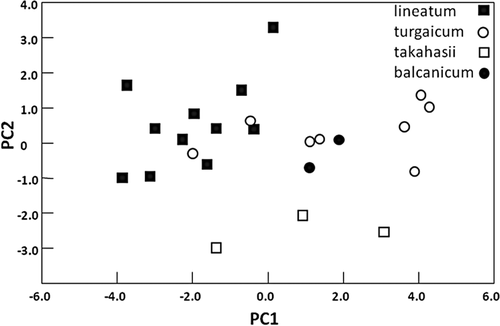
Principal components (PC) analysis of the lengths of all gill filaments and ratios of filaments 5 : 2 and 3 : 1 of four species of Simulium (Wilhelmia). The first PC is largely a linear measure of filaments, and the second PC is largely a measure of proportions.
Discussion
Our samples can be partitioned into four chromosomal segregates, which we recognize as separate species (S. balcanicum, S. lineatum, S. takahasii, and S. turgaicum), each supported by unique cytogenetic characters and geographical distributions.
These four species can be distinguished chromosomally at a glance from two sympatric members of the subgenus Wilhelmia, S. equinum and S. pseudequinum Séguy, by the presence of a chromocentre (Weber & Grunewald, 1989; Chubareva & Petrova, 2008). Although ectopic pairing of centromeres occurs in some populations of S. equinum, it is not present in all nuclei, and the expanded centromere region of chromosome I in S. equinum contrasts markedly with the slender condition in S. balcanicum, S. lineatum, S. takahasii, and S. turgaicum. The positions of the bulge and ring of Balbiani also are diagnostic. In our four taxa, they are in the distal one-third of the arm when in the standard sequence, with the bulge proximal to the ring of Balbiani, whereas, in S. equinum and S. pseudequinum, they are subterminal, with the bulge distal, unless displaced by floating inversions (Weber & Grunewald, 1989). Simulium paraequinum, which also has a chromocentre, has the bulge and ring of Balbiani in the proximal one-third of the arm, nearer the centromere (Petrova et al., 2003).
Species status
Our material of S. balcanicum from Germany and Turkey almost brackets the geographical range, which extends from Italy (Rivosecchi, 1978) and our collection site in Germany eastward to the Krasnodar Territory of Russia (Grinchuk & Chubareva, 1974). Our sample from Bârza, Romania, is within 200 km of the type locality (Lakatnik) in the Balkan Mountains of Bulgaria. The chromosomes reveal unequivocally that S. balcanicum is a distinct species, with no evidence of cryptic species, across its range of approximately 2000 km. Polymorphisms unique to one or a few populations are in low frequency, typically less than 0.10 per site. Common inversions exhibit a longitudinal gradient of frequencies, with a north–south tilt, although whether the frequencies are driven by selection in specific environments or by restricted gene flow, or both, is unknown. Inversion-frequency gradients are common in a number of simuliid species, typically grading toward loss or fixation at the distributional periphery (Rothfels & Featherston, 1981), a pattern also documented in Drosophila (Stocker, Foley & Hoffmann, 2004). We conclude that our samples of S. balcanicum are conspecific with the type specimen and with the types representing the three synonyms (secundum, danubiense, and severinense). The petiolate pair of pupal gills, originally described by Rubtsov (1956), provides the only diagnostic structural feature for S. balcanicum in any life stage (Crosskey & Zwick, 2007).
As S. balcanicum increases in prevalence eastward, S. lineatum diminishes in prevalence and is replaced by S. turgaicum. Where S. balcanicum overlaps geographically with S. lineatum, it typically occurs in different rivers. Our additional, albeit limited, findings of S. balcanicum in south-eastern Germany (G. Seitz, unpubl. data) suggest that it might be expanding its range westward.
Our German sample of S. lineatum probably represents the type specimen of S. lineatum; the type locality in Stolberg, Germany, is approximately 490 km north-east of our nearest sampling site (Landshut). Our English sample of S. lineatum is approximately 215 km south of the type locality of S. salopiense and approximately 1000 km west of our German sample. English and German populations differ only in the frequency of rearrangements and the presence of unique, rare inversions, which are not unexpected, given the geographical distance. We, therefore, agree with the synonymy of salopiense with lineatum originally proposed by Crosskey & Davies (1972). Although similarly having a high frequency (> 0.15) of the same inversions (IS-4, IS-6, IL-10, IL-11, IIL-8, IIL-9, IIL-11, and IIIL-5) as in England and Germany, the Corsica and Italian populations have fewer polymorphisms, perhaps reflecting their locations at the periphery of the distribution.
Our analyses provide no concrete evidence for cryptic species in S. lineatum s.s., nor did the analysis by Weber & Grunewald (1989) of populations in south-western Germany. Thus, the currently recognized synonymy (Adler & Crosskey, 2014) of falcula (Enderlein) with lineatum is valid. One caveat, however, is in order. Our English population of S. lineatum has the highest number of heterozygous inversions (N = 13) and of linked inversions (≥ 5) ever recorded for a single simuliid larva (Adler et al., 2010). The seven individuals with linked inversions in IS and in IIL might represent products of introgression, although we found no evidence of loose somatic pairing of homologues typical of hybrid individuals (Rothfels & Nambiar, 1981). The only other known species of the subgenus Wilhelmia in Britain are S. equinum and S. pseudequinum, and their chromosomal features are markedly different from those of S. lineatum. The possibility of cryptic taxa in S. lineatum will require additional sampling, particularly in England.
We resurrect the name turgaicum from synonymy with lineatum and apply it to our West Asian populations (Armenia, Iran, and Turkey) formerly known as S. lineatum. Rubtsov (1956) described S. turgaicum from Almaty, Kazakhstan, approximately 2500 km east of our nearest sampling site (Iran), and reported that the larvae and pupae occur in semidesert, small, warm rivers and irrigation channels, as well as cold rivers up to elevations of 2000 m in the Transcaucasus and Central Asia. Former Soviet studies (Grinchuk & Chubareva, 1974) consistently identified Armenian populations as S. turgaicum.
The chromosomal distinction between S. lineatum and S. turgaicum cannot be evaluated in sympatry. Our nearest samples are approximately 2100 km apart. Whether the distributions actually overlap is not known. If S. lineatum and S. turgaicum are sympatric, the zone of overlap probably lies in southern Russia or in the Balkans, perhaps Bulgaria, the easternmost reliable record for S. lineatum, based on the listing by Kovatschev (1979). One of the few putatively diagnostic features of S. turgaicum, the greater length of the anteriormost filaments of the pupal gill (Rubtsov, 1956), does not hold consistently. Some ecological distinction, nonetheless, might exist. Simulium lineatum is a lowland species, typically occupying habitats below 500 m a.s.l, whereas S. turgaicum is common at higher elevations (> 900 m). The altitudinal differences are reminiscent of sibling species in the S. ochraceum complex (Hirai et al., 1994). If the uniquely shared polymorphisms of S. balcanicum and S. lineatum are the result of common ancestry, rather than introgression, the species status of S. turgaicum is further supported by its relationship as sister to S. balcanicum plus S. lineatum, rather than to S. lineatum with which it historically has been in synonymy.
The full geographical range of S. turgaicum is unknown but is considered here to extend from Turkey eastward at least to the type locality in Kazakhstan. It probably extends well beyond Kazakhstan deep into Central Asia. Whether its range overlaps that of S. takahasii is unknown. Simulium takahasii occupies the eastern perimeter of the Palearctic Region in eastern China, Japan, and Korea (Adler & Crosskey, 2014); we consider the record of S. lineatum from Beijing (Adler & Crosskey, 2014) to apply to S. takahasii.
We continue to recognize S. takahasii as a valid species, primarily on pragmatic grounds (to maintain the current nomenclatural status) rather than on the basis of the few chromosomal differences from S. turgaicum. Whether S. takahasii is a valid species or conspecific with S. turgaicum suffers from the lack of an opportunity to test reproductive isolation in sympatry. Our collections of S. takahasii and S. turgaicum are separated by more than 7250 km. This geographical gap leaves open the question of whether some or all of the additional, putative species of S. (Wilhelmia), described from China and based on questionable structural characters, might be conspecific with S. turgaicum or S. takahasii, including Simulium germuense Liu, Gong, Zhang, Luo & An; Simulium pekingense Sun; Simulium qinghaiense Liu, Gong, Zhang, Luo & An; Simulium qingxilingense Cai & An; Simulium tachengense Mahe, An & Yan; Simulium tongbaishanense Chen & Luo; and Simulium xingyiense Chen & Zhang. Only the polytenes of S. xingyiense have been published (Huang et al., 2012), although the low-magnification resolution of the maps is not sufficient to allow comparison with our populations.
Chromosomal relationships
Our evaluation of the relationships of the four taxa is influenced by the choice of the standard banding sequence and by the scrambled sequence of the complement, which currently does not invite comparison with sequences of known taxa that could serve as outgroups. We can determine the polarity of only one relevant band sequence through outgroup comparison. Our standard sequence for IL-14 (sections 40–41) is equivalent to the standard sequence for the Simulium vernum group (sections 42C–44C, Brockhouse, 1985), the subgenus Simulium (sections 39c–41, Rothfels et al., 1978), and Simulium erythrocephalum (De Geer) (P. H. Adler, unpubl. data). If these taxa are taken as outgroups for this sequence, IL-14 of S. balcanicum is derived.
The chromosomes indicate that S. balcanicum and S. lineatum are more closely related to one another than either is to S. turgaicum. Although the relationships are not rooted, the same autosomal polymorphisms (e.g. IIL-8, IIL-11, and IIL-12) are uniquely shared regardless of the chromosomal sequence chosen as the standard; thus, the sister relationship of S. balcanicum and S. lineatum holds. The caveat, however, is that we do not know whether the shared rearrangements are primary (derived from a common ancestor) or secondary (acquired by introgression). Simulium balcanicum and S. lineatum are chromosomally most similar in the area of overlap (Germany), where they share five inversions.
On the basis of one uniquely shared inversion (IIIL-10), S. takahasii is most closely related to S. balcanicum. The relationship, however, might be an artefact of our choice of standard. In other words, if IIIL-10 is actually ancestral, our standard sequence for this inversion becomes the derived condition and the relationship of S. takahasii with S. balcanicum is not supported.
Pest problems
The attacks on livestock and humans along Turkey's Kizilirmak River (Yilmaz et al., 2007; Sariözkan et al., 2014) are the most severe recorded for any member of the subgenus Wilhelmia. Citizens in Isfahan, Iran, where we have cytotyped S. turgaicum, have complained about biting insects, although the situation is obfuscated by a failure to distinguish black flies from the common Culex mosquitoes in the area. Simulium lineatum is a pest of livestock and occasionally of humans in parts of Europe (Crosskey, 1990; Werner & Adler, 2005), and S. takahasii is a pest of cattle and horses in Japan (Bentinck, 1955). No pest problems have been reported for S. balcanicum, although the absence of reports might be related to the inability to distinguish the isomorphic females of this species from those of S. lineatum and S. turgaicum. Pest status of the group members is related to their shared propensity to feed on large mammals and to produce large populations in productive rivers and irrigation ditches near human and livestock foci. The Cappadocia outbreak probably represents an extreme example of these factors.
Geographical generalists and specialists
The existence of four discrete species, rather than one, reinforces a general pattern in the Simuliidae: wide distributions of putatively single species represent mosaics of geographically restricted cryptic species. This is not to say that all species of black flies with large geographical distributions are species complexes. Legitimate species with expansive ranges can reflect the ability to capitalize on anthropogenically altered environments (Adler & Kim, 1984). The trend, nonetheless, offers a guide for screening putative species for additional biodiversity. Accordingly, S. turgaicum, with the largest range of our four surveyed taxa and which has been minimally sampled, might consist of yet additional species.
The discovery that widespread black flies are composites of geographical specialists largely reflects the opportunity provided by the well-banded polytene chromosomes for whole-genome inspection of natural populations. The same trend is apparent in other groups of organisms that offer the polytene option, notably mosquitoes and drosophilids (Coluzzi et al., 2002; Rohde et al., 2006). This phenomenon, however, is not unique to black flies and other flies with high-quality polytenes. Variation in life-history aspects such as habitats, hosts, phenologies, mating behaviours, and communication systems have long signalled complexes of cryptic species (Mayr, 1963; Bickford et al., 2006). As genomic analyses become more powerful and routine, the opportunity for testing hypotheses of generalist species versus complexes of specialists will become more democratized.
Acknowledgements
We thank C. E. Beard for excellent technical assistance and C. L. Brockhouse and three anonymous reviewers for their insightful comments on the manuscript. This work was funded, in part, by National Science Foundation awards DEB-0841636 (Discovery and Prediction of Hidden Biodiversity in Black Flies (Diptera: Simuliidae)) under the American Recovery and Reinvestment Act of 2009 and DEB-0933218 (MIDGEPEET: A Collaborative Effort to Increase Taxonomic Expertise in Understudied Families of Nematocerous Diptera). Funding also came from The Scientific and Technological Research Council of Turkey Research Project 111 O 426 ‘The Molecular Classification of Black Fly (Diptera: Simuliidae) Species Which Pose a Problem in Central Kizilirmak Basin and the Investigation of Their Vector Potentials by Real Time PCR’, VEGA grant no. 1/0561/14 of the Ministry of Education of the Slovak Republic, and the Slovak Research and Development Agency under contract No. APVV-0436-12. This is Technical Contribution no. 6253 of the Clemson University Experiment Station, and is based on work supported in part by NIFA/USDA under project number SC-1700433.




