Latitudinal differences in temperature effects on the embryonic development and hatchling phenotypes of the Asian yellow pond turtle, Mauremys mutica
Abstract
The effect of incubation temperature on embryonic development and offspring traits has been widely reported for many species. However, knowledge remains limited about how such effects vary across populations. Here, we investigated whether incubation temperature (26, 28, and 30 °C) differentially affects the embryonic development of Asian yellow pond turtle (Mauremys mutica) eggs originating from low-latitude (Guangzhou, 23°06′N) and high-latitude (Haining, 30°19′N) populations in China. At 26 °C, the duration of incubation was shorter in the high-latitude population than in the low-latitude population. However, this pattern was reversed at 30 °C. As the incubation temperature increased, hatching success increased in the low-latitude population but slightly decreased in the high-latitude population. Hatchlings incubated at 30 °C were larger and righted themselves more rapidly than those incubated at 26 °C in the low-latitude population. In contrast, hatchling traits were not influenced by incubation temperature in the high-latitude population. Overall, 30 °C was a suitable developmental temperature for embryos from the low-latitude population, whereas 26 and 28 °C were suitable for those from the high-latitude population. This interpopulation difference in suitable developmental temperatures is consistent with the difference in the thermal environment of the two localities. Therefore, similarly to posthatching individuals, reptile embryos from different populations might have evolved diverse physiological strategies to benefit from the thermal environment in which they develop. © 2014 The Linnean Society of London, Biological Journal of the Linnean Society, 2014, 114, 35–43.
Introduction
Interpopulation variation in life-history traits is common in animal species that inhabit extensive geographical ranges (Denno & Dingle, 1981; Stearns, 1992; Iverson et al., 1993) and might reflect the combined effect of both genetic and environmental factors (Conover, Duffy & Hice, 2009). Identifying life-history variations among populations is crucial for understanding the life-history adaptation of an organism in response to distinct environments. Comparative studies on life-history strategies are common. However, most studies focus on postembryonic traits, such as offspring size, adult body size, and reproductive output (Litzgus & Mousseau, 2006; Ashton, Burke & Layne, 2007; Blanck & Lamouroux, 2007), with fewer studies focusing on embryonic traits (but see DiMichele & Westerman, 1997; Laugen, Laurila & Merilä, 2003; Du et al., 2010b). Various life-history strategies may be developed by the females and embryos of oviparous species to enhance embryonic development and survival under different environments. For example, females may adjust the seasonal timing of oviposition or modify nesting behaviours in response to climatic variation (Weisrock & Janzen, 1999; Kolbe & Janzen, 2002; Micheli-Campbell et al., 2013; Refsnider, Warner & Janzen, 2013). In addition, embryos shift optimal development temperatures or thermal tolerance in relation to environmental temperature variation (Mrosovsky, 1988; Jing & Kang, 2003; Ewert, Lang & Nelson, 2005; Doody et al., 2006; Liefting, Hoffmann & Ellers, 2009). Increasing evidence indicates that embryos are able to provoke behavioural and physiological responses to the changing environment, which challenges the traditional view that embryos are passive to their environments (Du et al., 2011; Du & Shine, 2014).
In oviparous species, developmental environments have important impacts on various embryo and offspring traits, such as embryonic development rate, hatchling size, and hatchling performance (Birchard & Deeming, 2004; Deeming, 2004; Booth, 2006). Temperature varies both temporally and spatially in nature. Consequently, it is one of the most important environmental factors that influence embryonic development and offspring phenotypes (Ackerman & Lott, 2004; Birchard & Deeming, 2004). A number of studies on reptiles have examined the effects of incubation temperature. The incubation period decreases nonlinearly as temperature increases, with eggs incubated at intermediate temperatures having relatively higher hatching success and producing higher-quality hatchlings than those at extremely high or low temperatures (e.g. Allsteadt & Lang, 1995; Rhen & Lang, 1999; Ji & Du, 2001; Warner & Andrews, 2002; Du & Ji, 2003; Booth, 2006; Brown & Shine, 2006). However, most of these studies have focused on a single population, with less effort being focused on interpopulation variations. The embryos of widespread oviparous species may experience distinct temperatures during development, although maternal behaviours, such as nest selection and nesting phenology, may compensate for geographical differences in the nest temperatures of some species (Kolbe & Janzen, 2002; Morjan, 2003; Ewert et al., 2005; Doody et al., 2006; Roedder, Kwet & Loetters, 2009). However, understanding geographical variation in the embryonic response to thermal environments has recently received increasing attention because of global climate-change issues (Oufiero & Angilletta, 2006; Du et al., 2010b; Sun et al., 2013), but more studies are needed.
The responses of reptilian embryos to incubation temperatures may vary among populations in widespread species as a result of local adaptation or phenotypic plasticity. Temperature-dependent differences might be present during embryonic development and in hatchling (offspring) phenotypes (Ewert, 1985; Du et al., 2010b). For example, embryos from high-latitude populations develop faster than their low-latitude conspecifics when incubated at an identical temperature (Ewert, 1985; Oufiero & Angilletta, 2006; Du et al., 2010b; Sun et al., 2013). In the Chinese skink, Eumeces chinensis, the optimal incubation temperatures for embryonic development differ between high-latitude and low-latitude populations and are assumed to match the local thermal environments (Ji et al., 2002). If these phenomena occur widely in populations of diverse species along a latitudinal cline (temperatures decrease as latitude increases), several hypotheses could be tested. First, the incubation period is expected to be shorter for high-latitude populations than for low-latitude populations. Second, hatching success is expected to be maximized at higher temperatures in low-latitude populations, and vice versa for high-latitude populations. Third, eggs are expected to produce high-quality hatchlings at high temperatures for low-latitude populations, and vice versa for high-latitude populations.
Here, we collected eggs of the Asian yellow pond turtle (Mauremys mutica) from two geographically distinct populations in China: the Guangdong (low-latitude) and Haining (high-latitude) populations. The eggs were incubated at three constant temperatures (26, 28, and 30 °C) to determine the effects of population origin and incubation temperature on hatching success, hatchling size, and the hatchling righting response. We used the assimilated data to test the three stated hypotheses and infer the potential implications on the life-history traits of these two populations.
Material and Methods
Study species
Mauremys mutica is an aquatic geoemydid turtle that is widely distributed in southern China, Vietnam, and Japan (the Ryukyu Archipelago) (Zhao & Adler, 1993). In China, M. mutica may be divided into two groups based on differences in morphological and genetic characteristics. Specifically, the low-latitude populations form one group that is distributed in Guangxi, Guangdong, Hainan, and Fujian provinces, whereas the high-latitude populations form a second group that is distributed in Anhui, Zhejiang, and Jiangsu provinces (Zhu et al., 2008). The average genetic distance between the southern and northern populations was found to be 0.305, according to the random amplified polymorphic DNA (RAPD) band patterns of 30 random individuals in each population (Zhu et al., 2008). Several studies have investigated the effect of incubation temperature on embryonic development, hatchling phenotype (e.g. size, sex, and locomotor performance), and posthatching growth in this species (Zhu et al., 2006a, b; Du, Wang & Shen, 2010a; Guo et al., 2010). Yet, interpopulation variation in embryonic responses to incubation temperatures remains unclear.
Egg collection and incubation
This species is extremely difficult, if not impossible, to find in its natural habitat in mainland China. We collected 84 (from 39 females) and 83 (from 34 females) fertilized eggs laid within a 2- to 3-day period from two private hatcheries in early June and mid-June of 2010, respectively. One hatchery was in Guangzhou (23°06′N, 113°15′E, Guangdong, southern China; hereafter, the low-latitude population) and another in Haining (30°19′N, 120°25′E, Zhejiang, eastern China; hereafter, the high-latitude population) (Fig. 1). The Guangzhou population belongs to the southern clade (Zhu et al., 2008), whereas the Haining captive population belongs to the northern clade (Chen et al., 2011). In both hatcheries, the captive populations were kept in outdoor artificial ponds (length × width: 20 m × 15 m) and thus were exposed to the local thermal environment. Female M. mutica lay multiple clutches of elongate eggs from April to August (Zhao et al., 2008). Thus, egg incubation may extend from May to September based on the incubation period of about 2 months at 28 °C (Du et al., 2010b). During this period, Guangzhou has higher mean soil temperatures (29.2 °C vs. 26.0 °C, Fig. 2) than Haining (http://cdc.cma.gov.cn).
Map of mainland China (top left corner) and a higher-magnification map of the boxed area showing the two sampling sites where Mauremys mutica eggs were collected.
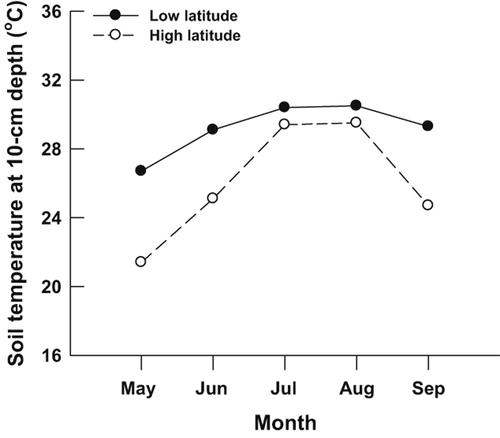
Monthly mean 10-cm-depth soil temperatures from May to September over a 15-year period (1998−2013) at the two sites where Mauremys mutica eggs were collected.
The collected eggs were numbered and weighed on a Mettler balance (±0.001 g), and then incubated in plastic containers (25 cm × 20 cm × 10 cm) containing known amounts of vermiculite and water at approximately −220 kPa water potential [dried vermiculite/water = 1:1 (wt/vol)]. A total of 21 containers, each containing nine or ten half-buried eggs, were placed in three incubators (Ningbo Life Science and Technology Ltd, Ningbo, China) at constant temperatures of 26 °C (25 and 28 eggs from the low- and high-latitude populations, respectively), 28 °C (29 and 24 eggs), and 30 °C (30 and 31 eggs). Eggs from a single clutch were randomly assigned to different temperature treatments to minimize the family effect, and the containers were moved among shelves twice a week, according to a predetermined schedule, to minimize the potential effect of thermal gradient within incubators. We added water to the vermiculite every week to compensate for water lost from evaporation and as a result of absorption by the eggs.
Hatchling traits
The incubation period was calculated as the number of days between oviposition and hatching. Upon emergence, each hatchling was weighed to determine body mass (±0.001 g), with carapace length and width measurements being taken after the carapace expanded to its normal shape (±0.1 mm). On the second day after hatching, we assessed the righting response of each hatchling in a temperature-controlled room at 28 °C. Each hatchling was placed upside down in an open area (250 mm × 200 mm × 40 mm) and its performance was recorded using a digital camera (DCR-SR220E; Sony, Minokamo city, Japan). Each hatchling was tested five times, and the time to right itself (defined as the time required for a hatchling to right itself after it began to move) was determined a posteriori from the videotapes (Delmas et al., 2007). If a turtle did not right itself within 10 min, the corresponding data were excluded from the statistical analysis.
Statistical analysis
All statistical analyses were performed with Statistica 6.0 (StatSoft, Inc., Tulsa, USA). Before parametric analyses, all variables were tested for normality using the Kolmogorov–Smirnov test, and for homogeneity of variances using Bartlett's test. Log-likelihood ratio tests (G-test) were used to detect the differences in hatching success between temperature treatments and populations. Linear regression analysis was used to determine the relationship between hatchling traits (body mass, and carapace length and width) and initial egg mass. Analysis of covariance (ANCOVA) (for hatchling body mass, and carapace length and width, using initial egg mass as the covariate) or analysis of variance (ANOVA) (for righting time) was used to examine differences in hatchling traits, with incubation temperature and population of origin as the fixed factors. Tukey's test was used for post-hoc multiple comparisons among the temperature treatments.
Results
Incubation period was independent of initial egg mass in all treatments (all treatments: P > 0.115) but decreased with increasing incubation temperatures in the two populations (F2, 133 = 994.90, P < 0.0001) (Fig. 3). The between-population difference in incubation period was dependent on incubation temperature (F2, 133 = 8.10, P < 0.001). At 26 °C, the incubation period was shorter for the high-latitude population than for the low-latitude population (F1, 42 = 10.60, P < 0.01). At 30 °C, the incubation period was longer for the high-latitude population than for the low-latitude population (F1, 49 = 10.11, P < 0.01). At 28 °C, there was no significant difference in incubation period between the two populations (F1, 42 = 0.10, P = 0.75) (Fig. 3).
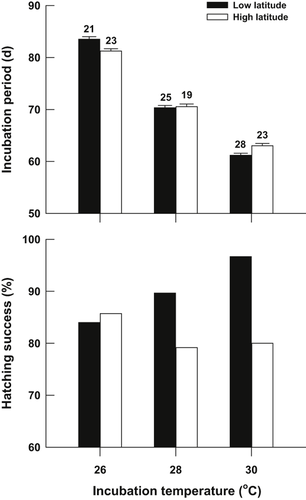
Hatching success and incubation period for Mauremys mutica eggs that were collected from low-latitude and high-latitude populations and incubated at three constant temperatures. Data on the incubation period are expressed as mean ± standard error (SE). The number at the top of each bar represents the sample size.
With increasing incubation temperature from 26 °C to 30 °C, hatching success increased in the low-latitude population, but slightly decreased in the high-latitude population (Fig. 3). Although the effect of incubation temperature on hatching success was not statistically significant in either population (G-test, both P > 0.10), hatching success was higher in the low-latitude population than in the high-latitude population when eggs were incubated at 30 °C [G = 4.43, degrees of freedom (d.f.) = 1, P < 0.05) (Fig. 3).
Hatchling body mass and size (determined by measuring the carapace length and width) were dependent on initial egg mass in each combination of population and temperature treatment (all treatments: P < 0.05). Overall, hatchling body mass was significantly affected by incubation temperatures (F2, 132 = 8.04, P < 0.001), but did not differ between populations after accounting for initial egg mass (F1, 132 = 2.69, P = 0.103). However, the effect of incubation temperature on body mass varied between the two populations (F2, 132 = 6.56, P < 0.01). Hatchling body mass was significantly affected by incubation temperature in the low-latitude population, with larger hatchlings being produced at 30 °C than at 26 and 28 °C (F2, 61 = 15.78, P < 0.001), whereas this trend was not observed in the high-latitude population (F2, 36 = 0.54, P = 0.587) (Fig. 4). Both incubation temperature and origin of population affected carapace length (temperature: F2, 132 = 7.04, P < 0.01; population: F1, 132 = 32.96, P < 0.0001; temperature × population interaction: F2, 132 = 1.56, P = 0.214) and carapace width (temperature: F2, 132 = 3.01, P = 0.053; population: F1, 132 = 17.91, P < 0.0001; temperature × population interaction: F2, 132 = 1.30, P = 0.276). Hatchlings from the low-latitude population had larger and wider carapaces than those from the high-latitude population. In the low-latitude population, the hatchlings produced at 26 °C were smaller than those produced at 30 °C (Fig. 4).
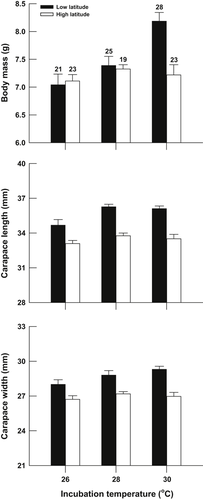
Body mass and size (carapace length and width) of Mauremys mutica hatchlings, from low-latitude and high-latitude populations, incubated at different incubation temperatures. Data are expressed as the adjusted mean + standard error (SE). Initial egg mass was used as the covariate in the analysis of covariance (ANCOVA) and was set at 12.8 g to calculate the adjusted means. The number at the top of each error bar represents the sample size.
The righting response of the hatchlings was significantly affected by incubation temperature in the low-latitude population, with the proportion of hatchlings that successfully righted themselves within 10 min increasing (G = 11.89, d.f. = 2; P < 0.01) and righting time decreasing (F2, 37 = 3.62, P < 0.05) as incubation temperature increased. However, this effect was not noticeable in the high-latitude population (proportion of righted individuals, G = 3.03, d.f. = 2, P > 0.10; righting time, F2, 52 = 0.51, P = 0.602) (Fig. 5). Relatively more hatchlings from the high-latitude population successfully righted themselves within 10 min, and took a shorter time to right themselves, than did those from the low-latitude population at 26 °C (proportion of righted individuals, G = 11.54, d.f. = 1, P < 0.01; righting time, F1, 20 = 13.58, P < 0.01) and 28 °C (proportion of righted individuals, G = 5.13, d.f. = 1, P < 0.05; righting time, F1, 30 = 4.43, P < 0.05) (Fig. 5), but not at 30 °C (proportion of righted individuals, G = 3.39, d.f. = 1, P > 0.05; righting time, F1, 39 = 1.33, P = 0.255).
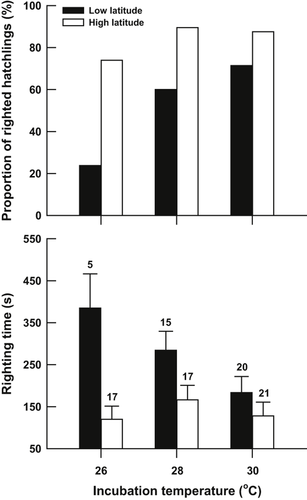
Righting response of Mauremys mutica hatchlings, from low-latitude and high-latitude populations, incubated at different incubation temperatures. Data on righting time are expressed as mean + standard error (SE). The number at the top of each error bar represents the sample size.
Discussion
Our study demonstrates that there are temperature-dependent differences in the embryonic development rate, hatching success, and hatchling phenotypes between low- and high-latitude populations of M. mutica. These results are generally consistent with the three stated hypotheses, although the response of embryonic development to temperature was more complicated than expected.
Consistent with our first hypothesis, a shorter duration of incubation was recorded in the high-latitude population than in the low-latitude population at 26 °C, but not at the two higher temperatures. Previous studies have shown that shorter incubation periods occur in high-latitude populations than in low-latitude populations for reptiles (Ewert, 1985; Oufiero & Angilletta, 2006; Du et al., 2010b; Sun et al., 2013) and other animals, such as insects, fish, and amphibians (DiMichele & Westerman, 1997; Laugen et al., 2003; Liefting et al., 2009). This countergradient variation may cancel out the effects of environmentally driven thermal factors on the timing of hatching and therefore maintain a relatively constant timing of hatching among populations in wide-ranging species (Conover & Schultz, 1995; Du et al., 2010b). In contrast, other species exhibit co-gradient variation along latitudinal clines (Poykko & Tammaru, 2010). For example, Trachemys scripta elegans eggs from high-latitude populations had significantly longer incubation periods than did individuals from low-latitude populations (Tucker & Warner, 1999). In the case of M. mutica, we observed a temperature-dependent difference in the incubation period between populations. This difference was consistent with the countergradient variation at the low incubation temperature of 26 °C and with the co-gradient variation at the high incubation temperature of 30 °C (Fig. 3). The physiological mechanisms underlying geographical variation in the incubation period may involve the developmental stage of embryos at oviposition and the developmental rate of embryos during incubation (Du et al., 2010b; Sun et al., 2013). It would be interesting for future studies to identify whether similar physiological pathways cause the between-population differences in incubation period in M. mutica.
The hatching success results essentially support the second hypothesis. Hatching success increased in the low-latitude population, but slightly decreased in the high-latitude population, as incubation temperature increased. A significant between-population difference in hatching success was obtained at 30 °C, but not at any other temperature (Fig. 3). These results indicate that: (1) the thermal tolerance in embryonic survival differs between the two populations, with embryos from the low-latitude population being more resistant to high temperatures, leading to higher hatching success than the embryos from the high-latitude population; and (2) the range of suitable incubation temperatures differs between the two populations. This between-population difference is consistent with previous studies on this species, which exhibits higher optimal incubation temperatures for low-latitude populations than for high-latitude populations (Zhu et al., 2006b; Du et al., 2010a).
The sensitivity of hatchling body mass and righting response to incubation temperatures differed between the two populations. Consistent with the third hypothesis, the eggs of the low-latitude population produced heavier hatchlings with faster righting responses at the high incubation temperature (30 °C) than at the low incubation temperature (26 °C). In contrast, hatchling mass and righting response were independent of incubation temperature within the range of 26–30 °C in the high-latitude population (Figs 4, 5). Compared with individuals in the high-latitude population, the heavier hatchlings at 30 °C in the low-latitude population might be the result of a shorter incubation period, and therefore lower energy consumption during incubation. This phenomenon arises because incubation temperature influences the mass conversion efficiency of eggs through shifting embryonic metabolism (Booth, 1998; Zheng et al., 2006; Reid, Margaritoulis & Speakman, 2009). Alternatively, more material may be left behind in the eggs of the high-latitude population at low incubation temperatures. Hatchling phenotypes and hatching success are sensitive to incubation temperatures, indicating that: (1) embryonic development is probably more sensitive to temperatures in the low-latitude population than in the high-latitude population; and (2) high temperatures favour embryonic development in the low-latitude population.
In summary, our study found that between-population differences in hatching success and hatchling phenotypes are temperature dependent in M. mutica. The optimal temperatures for embryonic development are higher in the low-latitude population than in the high-latitude population, which indicates adaptation to the local thermal environment. Therefore, similarly to posthatching individuals, embryos of oviparous reptiles from different populations might have evolved diverse strategies to benefit from the thermal environment in which they complete development (Denno & Dingle, 1981; Warkentin, 1995; Colbert, Spencer & Janzen, 2010; Sun et al., 2013). In addition, maternal behaviours also provide an important mechanism for organisms to adapt to changing climatic conditions, and enhance the survival of embryos and the fitness of offspring (Mitchell, Maciel & Janzen, 2013, 2014). For example, females may shift the timing of nesting and actively select nest sites and depths with a suitable thermal environment for embryonic development to reduce the negative effects imposed by changing climatic conditions (Ewert et al., 2005; Doody et al., 2006; Telemeco, Elphick & Shine, 2009). Yet, a large knowledge gap remains regarding these strategies because of logistical difficulties or simply a lack of investigations. In the future, studies on both the ecological phenomena and underlying physiological and biochemical mechanisms are required to understand how embryos are adapted to their environments.
Acknowledgements
We thank L. Wang, B. Sun, B. J. Sun, and W. Q. Tang for their assistance in the laboratory. We are grateful to three anonymous reviewers for their constructive comments on the manuscript. This work was supported by grants from the Natural Science Foundation of Zhejiang Province (No. Y3110276) and the Scientific Research Program of Hangzhou City (No. 20110332H04).




