Will We Soon See a Low Cost, Small Portable Device for the Therapeutic Monitoring of Lithium? An Implementation Science Investigation
Funding: This research was funded by Universidad de La Sabana MED-355-2023.
1 Introduction
Lithium is a first-line medication for the treatment of bipolar disorder [1], and is included on the World Health Organization list of essential medicines [2]. Despite this, lithium use is declining in North America and Europe [3]. The need to achieve a narrow therapeutic range means that some clinicians and patients opt for other alternatives. Currently, lithium therapeutic drug monitoring (TDM) is a barrier for new patients, increases patient burden, is stigmatizing, and may contribute to treatment noncompliance. In some rural and remote locations, and outside of major urban centres in developing economies, lithium TDM may not be available and this essential medicine cannot be prescribed. Recent technological advances indicate the potential to develop a small portable device for the TDM of lithium [4]. Our research group is currently developing a portable device for the TDM of lithium and has conducted focus groups to understand where this device is needed and what design of device will best serve that need (Figure 1). This project bridges the gap between technological innovations and the consumer by using an implementation science framework to explore barriers and facilitators to adopting this new technology.
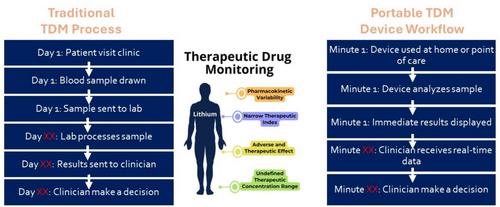
1.1 Focus Group
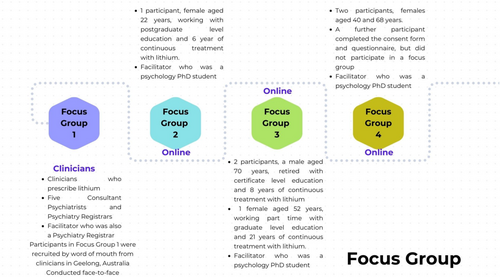
Focus groups were used to gather information about experiences and attitudes toward lithium TDM from clinicians who have prescribed lithium to their patients and patients treated with lithium. Face-to-face focus groups were preferred, but video conferencing participants were included to increase the sample size. Focus groups were recorded and transcribed for analysis using NVivo software. Four files were coded to 12 top-level codes and 7 s-level codes. Figure 2 presents the description of the four focus groups that participated in the study.
2 Results
The Group 1 video (clinicians) was a 37-min recording, and the Group 2 video was 33 min, Group 3 was 54 min, and Group 4 was 50 min. The codes commonly mentioned were concerned with the design of the novel device, including cost, features, and what information the device would display. Codes referencing toxicity, adverse events, and being outside of the therapeutic window were also commonly mentioned.
I'd get my bloods done, not realising they're not checking my lithium, so then I'd have to go back.
In a country town it's like a 24-hour day you have to plan to actually get [lithium levels] done.
I go hiking into the forest and stuff, and just to know before I leave, where are my levels sitting. Do I need to watch how much water, do I need to watch my sweating—all that kind of stuff. Same as even driving, because sometimes I'll shake so bad that driving is a bit difficult, and I know that's because my lithium levels are too high or something like that.
Routines, when they're messed up with the holidays or Christmas, I'd definitely use [a lithium TDM device].
More control in the day-to-day about your life and your changes, so you can continue with your lifestyle adjustments and trying new things.
We could just find out straight away—no wait lists, no barriers—it's really—yeah, it's exciting to know that could be a possibility.
I went toxic it was sort of out of the blue and I just started getting—I was very, very sick and my doctor said, hold on, you haven't done your lithium for a while, we'll go check that. It took a couple of days to get through and they then rang me and said, you need to come in now and you need to stop taking lithium.
It'd be amazing to be able to see your own results because I never really get to see my results. They'll just tell me, you're fine, you're in range.
I think you've got more power over it and power is control and you're not so reliant on other people if you've got that information in your hand. Maybe you could test yourself a bit more if you had a device and perhaps it would have prevented a toxic episode.
A dissenting option was expressed by one of the participants with lived experience, who felt that the current system worked well for her and change was not required. Several participants also mentioned that they would like the device to also measure liver and thyroid function.
2.1 Limitations
A limitation of the study was that no participants from remote locations, who may have greater disadvantages with the current system, were included in the study. Furthermore, patients who never commenced treatment with lithium because blood monitoring was either unavailable or considered too great a burden are unheard voices in this study. However, clinicians and researchers are well aware of patients who do not receive lithium treatment because of their geographic location or who need to relocate to major urban centres to receive treatment.
3 Discussion
A small portable device for the TDM of lithium is expected to be developed in the near future and several separate research groups may be currently working on this tool. Our study has suggested some key design features that may maximize the usefulness of the device, aid in making lithium available to more patients, and make the lived experience of lithium treatment less of a burden. Firstly, everything possible should be done to minimize the cost of the device. Some participants may suggest an increased range of functions for the device, but any impact on cost for these functions may negate their benefit. For example, it is not necessary that the device should also measure liver and thyroid function, as changes in liver and thyroid health occur slowly and an annual test is sufficient, whereas blood lithium levels can change suddenly. Adding these extra functions may be an unnecessary increase in device costs, and receiving results from other variables may be confusing to patients and create more stress by not knowing what to do with that data.
A further design challenge is that the small device will need to meet or exceed the specifications of accuracy, specificity, and sensitivity established by lithium TDM procedures conducted in an accredited laboratory, which is a challenging benchmark to achieve.
From the clinician point of view, other advantages of point of care devices were the opportunities to discuss with patients the reasons for low adherence when there are findings of sub-therapeutic levels, as well as the possibility of making instant decisions when levels are supra-therapeutic.
Further important design features include device robustness and ease of use. A non-invasive device or one that uses a pin-prick to obtain a blood droplet would be preferred. Many people are familiar with small devices used to monitor glucose levels in diabetes, and these devices have set an expectation that something similar should be available for the monitoring of lithium.
4 Conclusion
A small device for the TDM of lithium is currently in development and will be an important tool for people being treated with lithium. The technology for this device already exists. This device is wanted by patients and clinicians and may improve clinical care and access to lithium treatment. Scientists developing this device need to be aware of which design features will best serve our patients, taking care to avoid developing an expensive device with many features and to instead focus on a low-cost device that will benefit a maximum number of patients.
Acknowledgments
The authors thank the Universidad de La Sabana (MED-355-2023). Open access publishing facilitated by Deakin University, as part of the Wiley - Deakin University agreement via the Council of Australian University Librarians.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee of Barwon Health (approval number 92487, 26 June 2023).
Consent
The individual on whom this paper is written consented to participate in the research and has reviewed and approved this manuscript for publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data is available by request.




