The International Society for Bipolar Disorders Task Force report on pediatric bipolar disorder: Knowledge to date and directions for future research
Abstract
Objectives
Over the past two decades, there has been tremendous growth in research regarding bipolar disorder (BD) among children and adolescents (ie, pediatric BD [PBD]). The primary purpose of this article is to distill the extant literature, dispel myths or exaggerated assertions in the field, and disseminate clinically relevant findings.
Methods
An international group of experts completed a selective review of the literature, emphasizing areas of consensus, identifying limitations and gaps in the literature, and highlighting future directions to mitigate these gaps.
Results
Substantial, and increasingly international, research has accumulated regarding the phenomenology, differential diagnosis, course, treatment, and neurobiology of PBD. Prior division around the role of irritability and of screening tools in diagnosis has largely abated. Gold-standard pharmacologic trials inform treatment of manic/mixed episodes, whereas fewer data address bipolar depression and maintenance/continuation treatment. Adjunctive psychosocial treatment provides a forum for psychoeducation and targets primarily depressive symptoms. Numerous neurocognitive and neuroimaging studies, and increasing peripheral biomarker studies, largely converge with prior findings from adults with BD.
Conclusions
As data have accumulated and controversy has dissipated, the field has moved past existential questions about PBD toward defining and pursuing pressing clinical and scientific priorities that remain. The overall body of evidence supports the position that perceptions about marked international (US vs elsewhere) and developmental (pediatric vs adult) differences have been overstated, although additional research on these topics is warranted. Traction toward improved outcomes will be supported by continued emphasis on pathophysiology and novel therapeutics.
1 INTRODUCTION
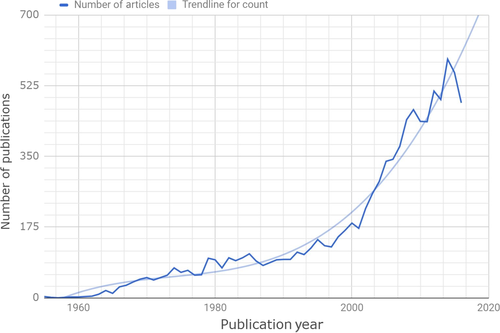
Over the past two decades, there has been tremendous growth in the scientific literature regarding bipolar disorder (BD) among children and adolescents (ie, pediatric BD [PBD]) (Figure 1). The literature now contains numerous gold-standard clinical trials of pharmacologic agents for mania, an increasing evidence base for adjunctive psychosocial treatments, several large-scale prospective clinical cohort studies, representative epidemiologic studies (particularly focused on adolescents) with international representation, numerous neurocognitive and neuroimaging studies, and an accelerating number of biomarker studies. Despite the volume, quality, and international spectrum of available literature, and regardless of the general consensus about some of the field's previously most divisive and controversial topics, there remains a perspective in the popular press, other branches of medicine, and even within mental health that the field of PBD lacks evidence and is replete with controversy. Therefore, the primary purpose of this article is to distill the extant literature, dispel myths or exaggerated assertions in the field, and disseminate clinically relevant findings. In this paper, an international group of experts completed a selective review of the extant literature (including PubMed and Web of Science/PsycINFO searches, querying other databases or conference proceedings and unpublished reports on an ad hoc basis), emphasizing areas of established findings and consensus, identifying limitations and gaps in the literature, and highlighting future directions to mitigate these gaps.
2 EPIDEMIOLOGY
2.1 Established findings and consensus
In community samples around the world, it is now well established that bipolar spectrum disorders—an umbrella that covers bipolar I disorder, bipolar II disorder, cyclothymic disorder [CycD], bipolar disorder not otherwise specified (NOS) and other specified bipolar and related disorders (OS-BRD)—occur in youths. The update of a published meta-analysis of epidemiologic studies1 using an identical search strategy identified six new studies satisfying inclusion criteria. The update includes 17 studies (seven from the USA) with 31 443 youth aged 7-21 years, 576 of whom met criteria for bipolar spectrum disorders.2-17 The updated weighted average prevalence rate of bipolar spectrum disorders is 2.06% (95% confidence interval [CI] 1.44%-2.95%).
There was substantial heterogeneity across studies, but it was not explained by year of data collection, lifetime prevalence (vs other time periods), or sex. Studies that included more adolescents had significantly higher prevalence estimates. Differences in the definition of BD explained the largest portion of variance between studies. As expected, broader definitions (ie, including NOS or CycD) had the highest rates while narrow definitions (ie, BD-I and -II only) had lower rates, but even this was not a complete explanation. The weighted average prevalence of BD-I was 0.49% (95% CI 0.22%-1.09%), with four of 12 studies reporting no BD-I cases. Although there is no epidemiologic study on PBD in Asia, several studies from clinical samples reported that PBD is also prevalent in Asia.18-20
Community rates are not higher in the USA, nor do rates appear to be increasing over time. In contrast, billing and services data show a marked increase in rates of diagnoses in the USA over a 20-year period.21, 22 Differences in training, conceptualization of cases and insurance demands appear more of a factor23 than underlying differences in prevalence.
In summary, PBD prevalence rates appear relatively stable across studies. PBD is more common than autism or schizophrenia and much less common than depression or attention deficit hyperactivity disorder (ADHD) in the community; more data are available with post-pubertal samples, and they find higher rates than before puberty.24
2.2 Limitations, gaps, and future directions
New epidemiologic studies need to systematically assess hypomania and mania and differentiate them from other non-mood psychopathology. Even the newest epidemiologic data were collected at least 10 years ago (range 1986-2005), and fewer than 1% of indexed papers on pediatric epidemiology include data about BD.1 Documenting age of onset of the first mood episode of each type will be vital, with more studies including prepubertal children, to clarify onset and course. If depression manifests first, it may not be clear that it is following a bipolar course until later. Onset age may be bimodal25, 26 and American youth may be at higher risk for very early onset.27 Existing literature confounds age and informant effects: studies with older participants are less likely to include interviews of parents. Studies relying only on retrospective self-report are likely to underestimate hypomania or mania, as people forget or minimize the events, whereas depression may be more salient. Using more consistent criteria across studies will reduce the largest source of variance identified in extant meta-analyses.
3 INTERNATIONAL FINDINGS
3.1 Established findings and consensus
Prior studies in adults suggested that, although BD occurs worldwide, there could be international differences in the clinical characteristics of BD, including age of onset, comorbidity, early adversity, and familiality.28, 29 Although international differences in BD are less well studied in youth as compared to adults, there now are PBD data from multiple countries including Australia/New Zealand, Brazil, China, France, India, Italy, South Korea, Spain, the Netherlands, Turkey, and the UK.30 In addition to the epidemiologic studies reviewed above, international BD data now include phenomenological,19, 31-33 comorbidity,19, 34 longitudinal,35, 36 treatment,37, 38 neuroimaging and biomarker,39-41 and high-risk studies.42, 43 Numerous commentaries discuss purported international differences in the prevalence of PBD, which may be attributable to including more subtypes,1 differences in the interview method,44 training,23, 45 or the type of sample studied (predominantly manic, depressive, treatment-seeking or registry), or a combination of these.46
Some findings converge with US data. For example, Brazilian families with PBD also show lower positive regard and higher negative expressed emotion (EE) compared to control families.47 There are, however, examples of non-replication. In contrast to US data, a recent Brazilian imaging study did not find white matter microstructure differences between PBD youth, healthy bipolar offspring and healthy controls,48 and parent report of youth mood symptoms showed much lower discriminative validity than parent report in other countries.49 However, it is unclear whether this a true international difference or a difference owing to methodologic factors.
A recent study directly compared Dutch and US samples of offspring of parents with BD.50 Both projects used the Achenbach Child Behavior Checklist (CBCL)51 as well as similar semi-structured interviews (eg, the Kiddie Schedule for Affective Disorders and Schizophrenia).52 After controlling for age, rates of DSM-IV BD-I (2.2% in the US sample and 1.5% in the Dutch sample) and BD-II (2% and 1%, respectively) were similar. In contrast, other disorders were significantly more common in the US sample, including depressive disorders (13% vs 4% in the Dutch sample), anxiety disorders (31% vs 9%, respectively), ADHD (22% vs 8%, respectively), and disruptive behavior disorder (19% vs 6%, respectively). CBCL Externalizing scores were higher in the US sample (mean + standard deviation 15.0 + 9.7 vs 10.8 + 7.6, respectively, P = .03). However, Internalizing scores did not differ between samples (raw M ~ 15), despite higher rates of anxiety and depressive disorders in the US sample. In the US, fewer parents had BD-I, affected parents were younger at their own illness onset, parental substance abuse rates were higher, and rates of parental employment and youth living with both biological parents were lower. These stressors may have contributed to the greater rates of problems in the US sample. The differences also illustrate the importance of the psychopathology of the parent bipolar sample to understanding rates of offspring psychopathology. The internalizing findings also suggest that there could be differences in clinical interpretation of similar levels of pathology, even using semi-structured approaches.
3.2 Limitations, gaps, and future directions
Future work needs to determine whether there are meaningful international differences in the prevalence, phenomenology, course, treatment, and biology of PBD. Translating scales into multiple languages and pursuing cross-cultural validation are accelerating progress by using consistent definitions,50 revealing considerable continuity in phenomenology.53 Combining data sets internationally would help calibrate definitions and examine common risk factors.
4 CLINICAL CHARACTERISTICS, DIFFERENTIAL DIAGNOSES, AND COURSE
4.1 Established findings and consensus
4.1.1 Clinical characteristics
Interest in BD phenotypes spans decades. Over 25 years ago, the issue of severe chronic irritability as part of the bipolar spectrum polarized the field; this issue is now largely resolved.46, 54, 55 With regard to symptom presentations, one position held that elation and/or grandiosity was required for a diagnosis of BD in youth.56 There now is substantial consensus that chronic irritability, regardless of explosiveness or severity, is not sufficient for a diagnosis of BD, in contrast with formulations that focused on rages or severe aggression.57 However, irritability is commonly present in youth with BD. Counting irritability as part of the diagnostic criteria for a manic or hypomanic episode requires that the irritability either begins or significantly increases in intensity in conjunction with the presence of accompanying manic (DSM Criterion B) symptoms. Thus, a youth with BPD may have chronic irritability, but the diagnosis would not be made based on that mood state.58-61 Although severe irritability and emotional dysregulation are not proxies for BD, they are also not mutually exclusive of episodic, DSM-adherent BD. Irritability that waxes and wanes spontaneously or in conjunction with other episodic changes in energy or sleep is much more suggestive of a mood disorder than other pathology.
While calls to include severe, chronic irritability as a developmental type of BD among youth have waned, substantial data have accrued concerning repeated brief discrete episodes of hypomania for less than 4 days as part of the BD spectrum. As among adults,62 youth manifest CycD63 and episodic OS-BRD, as well as BD-I and BD-II. However, as described below, youth with brief but well-defined episodes of mania or hypomania are at very high risk to switch into BD-I or BD-II (particularly if they have a family history of BD),64 and they have as much psychosocial impairment, suicidality, comorbidity (eg, substance abuse), and family history of BD as those with BD-I.65 Less research has specifically addressed CycD, which is often subsumed within the BD-NOS category due to lack of operationalized criteria.1, 63, 66
Ultra-rapid or “ultradian” cycling also has been associated with pediatric (as well as adult) BD.67 Although youth with BD, particularly younger children, often have more mood fluctuations than adults, it appears that “ultra-rapid cycles” describes mood fluctuation within an episode, and they are not themselves distinct episodes.68
Similar to adult BD, PBD has extensive comorbidity, both in representative epidemiologic studies with largely untreated samples17, 69, 70 and in clinical samples.71 With the exception of developmental disorders like ADHD and autism spectrum disorders, the patterns of comorbidity are broadly consistent between youth and adult presentations.71, 72 Apparent differences may partly reflect limited assessment of ADHD and autism spectrum disorders in adult samples rather than a true difference in comorbidities between these age groups, although some studies also suggest an association between ADHD and earlier age of onset of bipolar illness in adult samples.73, 74 The presence of comorbid disorders worsens the course and outcome of PBD, indicating the need for their identification and treatment. When determining comorbid diagnoses, it is important not to “double-count” overlapping diagnostic symptoms (eg, hyperactivity in ADHD and irritability in multiple disorders), as this leads to artificially high rates of comorbidity. Identifying discrete mood episodes and ensuring that overlapping symptoms are (i) over and above what can be expected from the individual's baseline and (ii) concurrent with mood episodes helps disentangle clinical presentations. Conversely, symptoms that persist in periods of euthymia suggest a non-mood etiology, and thus a “true” comorbidity. Finally, there is increasing evidence that medical comorbidity is relevant to PBD, both in terms of the association of physical health characteristics such as obesity with suicidality, neurocognition, and other markers of psychiatric symptom burden, and in terms of future premature cardiovascular disease.75-79 Medical comorbidity is closely related to both the mood symptoms and related lifestyle behaviors such as sedentary lifestyle and binge-eating in PBD.80, 81
4.1.2 Differential diagnosis
Diagnosing PBD requires changes in mood and behavior that are uncharacteristic of the individual and more extreme than developmentally appropriate, persist long enough to satisfy duration criteria, and have a clear impact on functioning. If comorbid disorders are present, mood symptoms need to be above and beyond the regular symptoms for the other disorder. For example, if symptoms of ADHD get worse episodically, and during these episodes the youth presents symptoms more specific to mania (eg, decreased need for sleep), a PBD diagnosis should be considered. Symptom sequence may help differentiate BD from some other disorders: a teen with BD might first experience symptoms of hypomania and depression and then begin using substances, in contrast to another teen who experimented with drugs first and then began showing irritability. However, it can be difficult to separate onset of a comorbid disorder from onset of manic/hypomanic symptoms.
Although the new and controversial DSM-5 diagnosis of disruptive mood dysregulation disorder (DMDD) cannot formally be diagnosed if the youth has BD,82 the phenotype commonly overlaps with those of all BD subtypes60, 83, 84 as well as other conditions, particularly oppositional defiant disorder (ODD).85, 86 Thus, one must evaluate the potential presence of BD even if DMDD is identified, and vice versa. The reliability of DMDD diagnoses was low in the DSM-5 field trials, suggesting that it may be challenging to accurately identify clinically.
4.1.3 Course
Multiple longitudinal studies have prospectively followed cohorts of youths for 4 or more years. Consistent findings include (i) high rates of progression from BD-NOS or CycD to BD-I or BD-II (eg, 43%),64 particularly in youth with a family history of BD; (ii) high rates of recovery from episodes, particularly with treatment (eg, 81.5%);87, 88 (iii) high rates of recurrence of depression, hypomania, or mania (eg, 62.5%);64, 87, 89, 90 and (iv) patterns of comorbidity91 and treatment response congruent with adult BD.92 The prodrome of BD-I or BD-II, often identified retrospectively in adult studies, commonly involves attenuated mood symptoms; and sleep disturbance may be a marker clinically (see reference for detailed discussion of prodrome).93
4.2 Limitations, gaps, and future directions
Remaining controversies include whether ADHD might be a prodrome for BD. Some studies suggest that ADHD might be a prodrome,94, 95 although some used interviews that blurred the distinction between mood episodes and more chronic presentations.44 Longitudinal studies of ADHD cohorts tend to find no or only small increases in risk of BD.96 Adding the DMDD diagnosis to DSM-5 created several gaps in terms of evidence-based assessment methods to differentiate DMDD from mood disorders, and lack of clarity regarding effective treatments. DMDD has its own definitional problems, as irritability includes both mood (often loses temper) and behavior (what happens when temper is lost).46 The next version of ICD will not add DMDD as a diagnosis, instead making a mood disturbance specifier for ODD.97 Research needs to evaluate the relative effectiveness of psychosocial (eg, parent training; emotion regulation strategies) and pharmacologic interventions for DMDD.61, 98 Longitudinal data also raise interesting questions about the possibility of remission of DMDD and bipolar disorders,55, 99 a topic that warrants further study.
5 MEASUREMENT
5.1 Established findings and consensus
There has been a large increase in the number of scales available and supported by data to measure different aspects of PBD. Myths ready for retirement include: (i) there is no validated rating scale or checklist for assessing manic symptoms in youth, (ii) a profile of scores on the CBCL51 can be used as a proxy for a diagnosis of PBD, and (iii) thinking in polarized terms about the value of a particular informant perspective in the evaluation of PBD (see Table 1). Common (and mistaken) beliefs include “teacher report is essential for confirming a diagnosis of mania,” vs “teacher report is useless in the evaluation of PBD”, or “parents’ perspective is always right” vs “parents’ perspective is hopelessly contaminated by their own mood or agenda.”
| Area | Myths | Data | Five-year needs |
|---|---|---|---|
| 1. Epidemiology |
|
|
|
| 2. International perspectives |
|
|
|
| 3. Clinical characteristics, differential diagnoses, and course |
|
|
|
| 4. Measurement |
|
|
|
| 5. Course, outcome, and comorbidity |
|
|
|
| 6. Pharmacologic treatment |
|
|
|
| 7. Psychosocial treatment |
|
|
|
| 8. Imaging and neurocognition |
|
|
|
| 9. Biomarkers |
|
|
- ADHD, attention deficit hyperactivity disorder; BD, bipolar disorder; BMI, body mass index; CBCL, Achenbach Child Behavior Checklist; CD, conduct disorder; CIDI, Composite International Diagnostic Interview; DMDD, disruptive mood dysregulation disorder; EE, expressed emotion; NOS, not otherwise specified; PBD, pediatric bipolar disorder; ODD, oppositional defiant disorder; OS-BRD, other specified bipolar and related disorders; SGA, second-generation antipsychotic; SMD, severe mood dysregulation; WHO, World Health Organization.
5.1.1 Current state of assessment
The current generation of practitioners completed their training before the bulk of research on PBD was available. Consequently, agreement between clinicians about the presence or absence of PBD in a particular case is poor.100, 101 Agreement also tends to be worse around CycD and OS-BRD,23, 102 which is unfortunate given that these may be more common than BD-I.1, 66
5.1.2 Cross-informant report on checklists
Multiple studies have evaluated parent, youth, and teacher report on both broad symptom checklists, such as the Achenbach System of Empirically Based Assessment,51 and manic symptom scales. Agreement between informants on ratings of the youth's mood symptoms tends to be only “fair,” ie, in the r = .2 to .3 range, consistent with meta-analyses of agreement about youth mood and behavior in general.103
Checklists can detect cases needing more in-depth evaluation for potential PBD. A recent meta-analysis found 63 effect sizes—38 based on caregiver, 14 on youth, and 11 on teacher report, with eight checklists providing at least two different effect sizes. Teacher and youth reports had medium effect sizes, and parent/caregiver reports had the largest effect sizes. The Achenbach Externalizing Scale outperformed the putative “bipolar” profile, consistent with findings that the “bipolar” profile is not specific to PBD.104, 105 Manic symptom scales fared better at identifying PBD, with three top tier options: The Parent General Behavior Inventory106 and its 10-item mania short form,107 the Child Mania Rating Scale108 and its 10-item short form,109 and the parent version of the Mood Disorder Questionnaire.110
These scales are not accurate enough to justify their use as universal screening devices or as substitutes for a (semi-)structured diagnostic interview. However, they can change the probability of a PBD diagnosis within an evidence-based medicine or Bayesian decision-making framework111, 112 and substantially improve the accuracy of clinical interpretation and agreement about next clinical action.102, 112 Some checklists have also shown sensitivity to treatment effects in masked randomized controlled trials (RCTs), with effect sizes equal to those based on direct interview of the youth and primary caregiver.113, 114 Checklists can improve diagnostic decision making in a range of clinical settings, as well as measuring treatment response.90, 100-102
5.1.3 Diagnostic and severity interviews
Semi-structured or structured diagnostic interviews that systematically evaluate mood symptoms remain the best available method to establish PBD diagnoses52, 115 as well as symptom severity. The Young Mania Rating Scale (YMRS)116 and Child Depression Rating Scale Revised117 have been the industry standard interviews to quantify severity. Newer alternatives were designed for pediatric patients, with developmentally appropriate anchors and complete coverage of DSM mood symptoms.118 Psychometric differences between severity interviews are small,119, 120 so the choice is more conceptual than statistical. Interview length and training requirements are barriers to routine clinical use.44 More structured methods are faster and easier to use consistently, and are surprisingly popular with patients.121 It is important to check for mood episodes in the context of developmental history at the outset of the interview. Few methods provide a clear algorithm for CycD or OS-BRD—a key omission given the data on prevalence and burden.
5.1.4 Risk factors as part of clinical evaluation
Risk factors inform the initial diagnostic evaluation process, and may guide treatment selection. Family history of mood disorders—and BD in particular—is the best-validated risk factor, recommended for routine clinical evaluation, while keeping in mind that the majority of youth with a family history will not necessarily develop BD. Brief screening tools make adding family history clinically feasible.122 High EE (ie, criticism, hostility, and emotional overinvolvement) and high family conflict are risk factors for treatment drop-out and more rapid relapse.90 High-EE families may show larger responses to family-focused psychotherapeutic interventions.123
5.2 Limitations, gaps, and future directions
Although considerable advances have been made in the use of rating scales, checklists, and structured diagnostic interviews, little work has been done with neurocognitive or neuroimaging methods using clinically generalizable comparison groups to establish whether these methods have diagnostic specificity. Productive next steps in assessment research would be to examine hybrid batteries that combine checklists and neurocognitive measures, biomarkers, or imaging to test incremental validity (ie, the degree to which each measure improves diagnostic accuracy) in order to guide the most cost-effective test sequences. Better assessment of developmental phenomenology would be valuable to clarify prodromal presentations and trajectories. Structured interviews could be redesigned to better capture duration, potential prodrome, and trajectories. Rapid changes in Internet-based and smartphone delivery of clinical assessments need to be integrated with traditional assessment and treatment. Finally, feasibility, acceptability and utility of translation of research tools into real-world measurement-based care need to be studied.
6 PHARMACOLOGIC TREATMENT
6.1 Established findings and consensus
This section summarizes extant literature about pharmacologic treatment of PBD, emphasizing RCTs. For additional detail, the reader is referred to recent reviews on this topic.37, 124-129
6.1.1 Manic/mixed episodes
Second-generation antipsychotics (SGAs) approved by the United States Food and Drug Administration (FDA) for the treatment of acute mania and/or mixed mania in children and adolescents (10-17 years) include risperidone, aripiprazole and quetiapine, olanzapine, and asenapine13-17 (http://www.accessdata.fda.gov/scripts/cder/daf/). Lithium has FDA indication for the treatment and recurrence prevention of mania in youths aged 12 years and older, bolstered by a recently published positive 8-week RCT for youths 7-17 years old with BD-I manic/mixed episodes.130 In that study, change in mania symptom scores was significantly larger in lithium-treated participants, as was global improvement at week 8, compared to placebo-treated participants.
A large (n = 153) 8-week National Institute of Mental Health (NIMH)-funded RCT compared lithium vs divalproex vs placebo in youths aged 7-17 years with BD-I mania.127 Participants randomized to divalproex had a significantly larger reduction in YMRS scores (ΔM = 8.3) from baseline compared to those randomized to placebo (ΔM = 5.3) (P = .04). There was not a statistically significant difference in YMRS scores for subjects randomized to lithium (7.2) compared to placebo (P = .19), or divalproex vs lithium (P = .35). However, another 4-week RCT of divalproex extended-release was negative when comparing an active group (n = 74) to placebo (n = 70).131 In this investigation, treatment with divalproex extended release (ER) promoted a mean reduction of 8.8 points in the YMRS, and the placebo group presented a mean reduction of mania of 7.9 points (P = .6). In an open extension of this trial with divalproex ER, where only 26 of 66 patients completed a 6-month follow-up, the mean YMRS score decreased by 2.2 points from a baseline of 20.3.
An early meta-analysis on the treatment of PBD (N = 2666 participants across 46 trials) reported that SGAs were highly efficacious and superior to the modest anti-manic effects for traditional mood-stabilizing agents, such as lithium carbonate, divalproex sodium, and carbamazepine, when used as monotherapy.129 Another meta-analysis of double-masked placebo-controlled RCTs of treatment for BD-I mania among youth (n = 1609) and adults (n = 6501) found that SGAs were more efficacious but more metabolically burdensome compared to traditional mood stabilizers among youth, but showed no such differences among adults.132 However, these meta-analyses predated recent studies supporting the efficacy of lithium.
The anticonvulsants divalproex sodium and carbamazepine have not fared well head-to-head with SGAs. In two meta-analyses, treatment effect was less robust with anticonvulsants than with SGAs.105, 106 An RCT of divalproex extended release was negative,131 as was a large RCT of oxcarbazepine,110 and a topiramate RCT in youth was interrupted prior to its completion due to negative results in adult studies; it failed to show efficacy in youth given inadequate power.133 A study of 10 outpatients with BD (11-17 years old) whose weight increased by > 5% during treatment with mood-stabilizer or antipsychotic monotherapy were switched to topiramate; reductions in manic symptoms and weight were observed.133, 134
In the “Treatment of Early Age Mania” 8-week trial, which included 290 youths aged 6-15 years with BD-I manic or mixed episodes, response rates were 68% for risperidone, 35% for lithium, and 24% for divalproex sodium.135 The advantage of risperidone vs lithium was greater for youth with ADHD (vs non-ADHD) and non-obese (vs obese) youth.136 However, rates of comorbid ADHD in this study were > 90%, limiting power for ADHD vs no-ADHD comparisons, and some sites showed similar response rates for risperidone and lithium. Concerns have been raised regarding several characteristics in this sample, including prolonged episodes, very early childhood onset, and high rates of rapid cycling, constraining comparisons with adult studies.137
6.1.2 Bipolar depression
The combination of olanzapine and fluoxetine has an FDA indication for BD depression in youth aged 10-17 years old.138 In a recently presented RCT of 347 youth 10–17 years old, lurasidone was associated with significantly greater improvement in depression symptoms at 6 weeks as compared to placebo (effect size, 0.45). 139 There have been two negative RCTs of quetiapine for bipolar depression.140, 141 In a sample of 193 adolescents (10-17 years old) with BD-I or BD-II who received quetiapine or placebo for 8 weeks, although nonsignificant differences were observed between the responses in the placebo and quetiapine groups (P = .63), high rates of response were observed in both groups (placebo: 55%, and quetiapine ER: 63%).127 A smaller trial of 32 adolescents (12-18 years old) with BD-I where subjects were randomized for 8 weeks to placebo (n = 15) or quetiapine (n = 17) revealed no statistically significant differences in response rates between the placebo (67%) and quetiapine (71%) groups.128 Positive preliminary open trials142, 143 or chart reviews144 of lithium and lamotrigine warrant future RCTs. However, high placebo response rates in depression studies require larger samples than mania trials or blinded placebo lead-in. Although the precise frequency of antidepressant-induced mania is uncertain,145, 146 practice parameters and guidelines suggest avoiding antidepressant monotherapy; this approach is supported by pharmaco-epidemiologic data.147
6.1.3 Maintenance/continuation treatment
There have been few maintenance/continuation treatment studies in youth, most of which have been previously reviewed.125 An early observational study of continuation treatment with lithium found an increased risk of relapse among adolescents who were not treated with lithium following hospitalization for mania.148 A subsequent study of children and adolescents with BD who had been stabilized on the combination of lithium and divalproex found a similar survival time to recurrence among those randomized to continuation treatment with monotherapy with lithium or divalproex.149 In a larger continuation study of 226 youth with BD (mean duration 124.4 days), YMRS scores decreased a further 12.4 points.150 In a 72-week maintenance study, 96 outpatient children, 4-9 years old, with PBD who had been stabilized on aripiprazole (mean dose 6.4 mg) were randomized to continuation with aripiprazole or placebo (n = 30/group).151 Although children randomized to aripiprazole, vs placebo, were enrolled significantly longer until time to discontinuation for a mood event or for any reason, the study was constrained by high drop-out rates within the first 4 weeks (50% for the aripiprazole group; 90% for the placebo group), understood by the authors as a nocebo effect. No significant between-group treatment effects on symptom rating or changes over time were observed for mania, depression, general impression, or global assessment. Another placebo-controlled continuation study of aripiprazole (10 mg or 30 mg) in youths (10-17 years old) with manic/mixed episodes followed participants for 26 weeks under double-blind conditions after a 4-week acute treatment phase.152 For both aripiprazole dosages, there were greater reductions in YMRS symptoms (in last observation carried forward analyses only) and time to all-cause discontinuation was longer for both aripiprazole dosages compared to placebo. There were also higher response rates, greater improvements in global functioning, and greater reductions in clinical global impression bipolar severity for each of the aripiprazole groups vs placebo. Another recent study of 301 children and adolescents with BD-I stabilized for at least 6 weeks on adjunctive lamotrigine, in combination with up to two mood stabilizers or antipsychotics, randomized participants to 36 weeks of either continuation treatment with lamotrigine or placebo.153 Although the primary analysis was negative, continuation with lamotrigine was superior to placebo (hazard ratio 0.46) in 13-17-year-olds, but not in 10-12-year-olds (hazard ratio 0.94). Although reasons for this discrepancy are uncertain, it may relate to higher rates of ADHD in the younger group. Finally, a 50-week open-label, flexible-dose extension study of 321 youth, 10-17 years old, with BD-I (lead-in study treatment: placebo, n = 80; asenapine, n = 241) reported that: (i) there was a mean 9.2-point reduction in YMRS at week 50; (ii) 79.2% were considered responders based on 50% reduction in YMRS relative to acute trial baseline; (iii) somnolence/sedation/hypersomnia (42.4%) and significant weight gain (34.8%) were very common.154
6.1.4 Comorbidity
Positive results have been reported regarding the treatment of comorbid ADHD with mixed amphetamine salts and methylphenidate, albeit in modest-sized samples, particularly in euthymic youth.37, 155, 156 Aripiprazole did not differ from placebo in ADHD symptom reduction.157 Preliminary findings from open trials and case reports suggest promise for adjunctive atomoxetine.158 Although stimulants, as well as antidepressants, hold some risk of precipitating mania, precise estimates of this risk are lacking, both with and without ongoing mood stabilizer cotreatment.145 A single small RCT found that lithium improved mood symptoms and comorbid substance use disorder among adolescents with, or at risk for, BD.159
6.1.5 Nutritional interventions
Four open-label nutritional trials, three of Omega 3 (Ω3) monotherapy (n = 3) and one of a commercially available multinutrient, in youth with BPSD provide preliminary evidence of potential benefits in terms of manic and depressive symptoms, global functioning, and parent-rated internalizing and externalizing behaviors.160-162 A 12-week pilot RCT (N = 23 youth with BD-NOS or CycD) found that the combination of Ω3 and psychoeducation was superior to placebo and active monitoring.163 An RCT of flax seed oil (alpha-linolenic acid [ALA]) in youth with BD-I/BD-II was negative.164
6.2 Limitations, gaps, and future directions
Positive RCTs for maintenance treatment and bipolar depression are lacking, although preliminary positive open trials hold promise. Pharmacologic treatment of comorbidities other than ADHD is another gap. Given concerns about heterogeneity of longitudinal course, it would be helpful to examine whether subtype of BD or age of onset moderates treatment response. Future studies should include neuroimaging, neurocognition, and peripheral biomarkers as moderators and predictors of treatment response. Large-scale RCTs of nutritional interventions are warranted, based on positive pilot RCTs and open-label trials. Studies concentrating on CycD and OS-BRD are crucial to address the need for titrated and evidence-based options, along with more masked maintenance studies to look at altering trajectories, and head-to-head trials and trials of combinations with non-pharmacologic treatments. Although systematically addressing the topic of offspring of parents with BD was beyond the scope of this article, it is important to note that there is also a need for large-scale RCTs examining the relative efficacy, tolerability, and safety of antidepressants vs mood stabilizers for anxiety and/or depression, and the efficacy, tolerability, and safety of stimulants for ADHD, among BD offspring.
7 PSYCHOSOCIAL TREATMENTS
7.1 Established findings and consensus
Adult psychotherapy trials provide substantial evidence for the effectiveness of family-focused therapy (FFT),165 group psychoeducation,166-168 and interpersonal and social rhythm therapy169 in improving symptoms, reducing rates of recurrence, and hastening recovery in adults with BD. In recent years, an accumulating body of evidence similarly supports the use of manualized psychotherapies for the treatment of PBD. A recent review highlighted 13 unique studies170 and three studies published since that review further strengthen the evidence base,171-174 whereas one additional study offers mixed evidence.175 The category of family psychoeducation plus skill building can be considered as a well-established treatment, meaning that two or more research groups have, via independent RCTs, demonstrated efficacy.176 Dialectical behavior therapy171 can be considered possibly efficacious, based on a single RCT or multiple studies by the same group, while interpersonal and social rhythm therapy177 remains experimental in youths at this time. Effectiveness trials suggest that family psychoeducation plus skill-building approaches have excellent acceptability and sustainability in community settings.178, 179
Treatment mediator analyses suggest that psychoeducation leads families to improve the quality of services they utilize, mediated by parental treatment beliefs; improved quality of services, in turn, mediates improved outcomes.180 Improved parenting skills and coping, family flexibility and family positive reframing are linked to improved mood and global functioning in youth with PBD.181 Treatment moderators include youth impairment182 and family EE,123 along with comorbid disorders, youth age, and family socioeconomic status (SES). Predictors of better treatment response include greater impairment, more stress/trauma history for the child, less Cluster B personality symptoms in parents,182 and comorbid anxiety disorders.131
With regard to secondary outcomes other than mood, family psychoeducation plus skill building are also associated with a reduction in behavioral symptoms.183 In an open trial of an adaptation of family psychoeducation and skill building, benefits were observed for adolescents with BD and comorbid substance use disorders.184 While no dismantling studies have been conducted, therapeutic ingredients common to the psychotherapy category family psychoeducation and skill building should be used. These include family involvement; psychoeducation about etiology, symptoms, course, medications, risk and protective factors, and effective treatment of BD; skill building (especially communication, problem-solving, and emotion regulation skills); and relapse and recurrence prevention.170
In regard to prevention, or delay of onset, two RCTs have examined the impact of family psychoeducation plus skill building in youth at high risk for developing BD. Youth who received FFT had more rapid recovery from their initial mood symptoms, experienced more weeks in remission, and had lower YMRS scores over 1 year compared to youth who received enhanced care.185 In a second study, youth with depression and transient manic symptoms were 4 times less likely to convert to BPSD at 12-month follow-up if they received multi-family psychoeducational psychotherapy plus treatment as usual (TAU) compared to the group who received only TAU.186
7.2 Limitations, gaps, and future directions
More work is needed to (i) determine the impact of low-risk interventions (eg, psychosocial treatments and nutritional interventions) in preventing or delaying the onset of BD in high-risk youth; (ii) test psychotherapies in diverse populations, including different SES and cultural backgrounds; (iii) determine what modifications are appropriate based on age or treatment format (eg, group or individual/family); (iv) determine the impact of psychotherapy on functional and quality of life outcomes as well as symptoms; (v) develop efficient ways to train large numbers of clinicians in these treatments; (vi) identify mechanisms of change and essential treatment components;170 (vii) examine the potential benefits of smartphone or Internet-based delivery; (viii) evaluate combinations of pharmacotherapy and psychotherapy; and (ix) determine how best to disseminate manual-based psychotherapies into health care settings.
8 NEUROIMAGING AND NEUROCOGNITION
8.1 Established findings and consensus
Prevailing pathophysiologic models of BD implicate abnormalities in brain regions that regulate emotion and attention, namely the emotional control network (ECN).187 The ECN is comprised of ventrolateral and ventromedial prefrontal networks, which modulate the limbic system, specifically the amygdala, in conjunction with subcortical nuclei, such as the thalamus and striatum.187, 188 Increasing evidence suggests that there are anatomical, neurochemical, functional and cognitive abnormalities within the ECN in PBD.188, 189 Pathophysiologic abnormalities underlying other cognitive functions that are altered in BD, such as reward processing, have also been studied.190
8.1.1 Neuroimaging
Brain structure
Within the ECN, the amygdala plays a central role in emotional regulation. Two meta-analyses of magnetic resonance imaging (MRI) studies reported that youth with PBD present with smaller amygdala volumes compared with controls.191, 192 One prospective study that evaluated youth after their first manic episode showed that amygdala volumes failed to show a normal increase with aging in patients, whereas this did not occur in controls or in youth with ADHD, suggesting that hampered neurodevelopment of the amygdala may underlie the dysfunctional emotional processing present in the ventral-limbic pathway.193 Diffusion tensor imaging (DTI) studies have investigated abnormalities in the white matter microstructure of youth with PBD. Preliminary findings suggest the presence of abnormal white matter microstructure in superior frontal regions,194, 195 and inferior/ventral frontal areas, such as the orbitofrontal or anterior cingulate cortex, and anterior regions of the corpus callosum196-200 compared to controls. One study found even greater reduction in white matter integrity in the anterior limb of the internal capsule in PBD vs adult BD.201 Most of these studies had very small sample sizes, but, together, they are consistent with a hypothesis of structural connectivity deficits between prefrontal-subcortical areas that underlie the prefrontal-limbic dysfunction in PBD.
Brain function
Meta-analyses of over 20 task-based functional MRI (fMRI) studies with approximately 500 youth concluded that BD involves hypoactivation of prefrontal areas and hyperactivation of limbic areas.202, 203 Weigbreit et al (2014) included more emotional (emotional face processing) than non-emotional (or cognitive) tasks whereas Lee et al (2014) did not discriminate the effects of emotional vs cognitive tasks. PBD presents with greater activation in the right amygdala, parahippocampal gyrus, and left putamen, and lower activation in the right ventrolateral and dorsolateral prefrontal cortices compared with controls in paradigms that involve emotional stimuli.202, 203 These findings suggest that prefrontal regions are unable to modulate hyperactive limbic regions,203 due either to a primary prefrontal dysfunction or to altered connectivity between prefrontal and limbic regions. Studies using non-emotional tasks (eg, reversal learning and reward anticipation) also showed abnormal functional activity of prefrontal areas, such as the dorsolateral prefrontal cortex, parietal regions, such as the temporal gyrus, and subcortical regions, such as the thalamus, during processing of the tasks.204-206 fMRI studies using tasks of attention and response inhibition have also found that prefrontal areas involved in attention and inhibitory control (namely the ventrolateral prefrontal cortex) are hypoactive while processing a continuous performance task in patients with PBD and comorbid ADHD, while posterior parietal and temporal areas are hyperactive, possibly as a compensatory mechanism.207 Other studies using continuous performance task and response inhibition also have found underactivation of the ventrolateral prefrontal cortex.208, 209 The few resting state fMRI studies in PBD show that youth with PBD present with fronto-temporal, amygdala-hippocampus and amygdala-precuneus functional connectivity alterations,210-212 with similar alterations observed during bipolar depression.213 Together, these studies suggest that PBD is characterized by abnormal prefrontal-limbic functional connectivity in the processing of emotion, attention, and reward. Although still too preliminary to allow any definitive conclusions about the neurophysiology of PBD, these findings converge with adult BD findings.
Brain chemistry
Proton magnetic resonance spectroscopy studies are particularly important to help understand two current neurobiological hypotheses of BD, ie, glutamatergic dysfunction and mitochondrial dysfunction or energy metabolism dysfunction. Several studies in PBD have shown abnormalities in glutamate or glutamine levels in prefrontal brain areas.214, 215 Abnormal levels of metabolites that are considered to be biomarkers of mitochondrial dysfunction or cell energy metabolism, such as decreased N-acetyl-aspartate or increased myo-inositol levels, were found in dorsal and ventral areas of the prefrontal cortex in PBD.216-224
Although very valuable given the possibility of in vivo measurement of metabolites that might relate to pathophysiologic processes of neuronal integrity/viability, glutamatergic neurotransmission, and cell energy metabolism, the exact meaning of these results is poorly understood, partly because they are difficult to replicate or to predict. For instance, although several proton spectroscopy studies reported positive results for specific metabolite differences between patients and controls (eg, N-acetyl-aspartate or glutamine),215, 220, 223, 224 within the same experiment, results are often negative for other metabolites (eg, creatine, myo-inositol and choline).215
8.2 Neuroimaging in relation to treatment
There is a preliminary literature regarding neuroimaging studies in relation to treatment of PBD. For instance, an association between change in prefrontal glutamate concentrations and change in manic symptoms was reported in patients who achieved remission of mania after treatment with divalproex sodium.225 Remission of mania after olanzapine treatment was associated with an increase in ventromedial prefrontal N-acetyl aspartate and choline levels.226 Quetiapine treatment for bipolar depression may lead to decreased neural activity in the left occipital cortex, and increased neural activity in the left insula, left cerebellum, and right ventrolateral prefrontal cortex during performance of a face emotion recognition task.227 Treatment of mania with ziprasidone was associated with an increase in activation in the right ventrolateral prefrontal cortex in response to a sustained attention task, which suggests that the ziprasidone antimanic effect is associated with improved prefrontal modulation of emotional regulation.228 Carbamazepine treatment of mania was also associated with increased activation in Brodmann area 10 of the right prefrontal cortex in a small sample of 11 adolescents,229 further suggesting that mood stabilizers might exert their beneficial effects by improving the top-down prefrontal modulation of limbic areas. Treatment response to risperidone and divalproex sodium for mania may lead to increased functional connectivity of the amygdala within the ECN.230 These preliminary findings offer insights regarding putative mechanisms of action of these medications. One recent study provided evidence that some psychotherapy modalities, such as FFT, improve dorsolateral prefrontal cortex activation and decrease amygdala activation in youth with mood dysregulation at familial risk for BD,231 which is consistent with the putative treatment effects of mood stabilizers (ie, improved prefrontal modulation of limbic areas, or better “top-down control”). Additional studies are necessary to better understand the physiology of treatment response and guide improved therapeutic strategies.
8.2.1 Neurocognition
PBD is associated with impairment in several cognitive domains.232 A meta-analysis of 10 cognitive studies reported overall cognitive deficits in PBD (n = 352) compared to healthy controls (HCs; n = 439) with mean effect sizes that are: large in verbal memory; moderate to large in attention and working memory; moderate in executive functioning, visual perceptual skills, and visual memory; and small to moderate in motor speed, reading achievement, full-scale IQ, and verbal fluency.232 A prospective study showed that youth with PBD may exhibit a delay in cognitive development compared with controls in some cognitive domains (eg, executive functioning and verbal memory), despite optimal pharmacologic management.233 Thus, it is essential to investigate therapeutic strategies, such as cognitive remediation234 and/or optimizing cardiometabolic health,78 that might be useful to restore these cognitive deficits and improve academic, social and functional impairments in youth with PBD.
In summary, in the last two decades, many neuroimaging and cognition studies have demonstrated anomalous brain structure, brain function, and cognition, alongside preliminary evidence of lagging neurocognitive development in PBD, mainly relating to the ECN.
8.3 Limitations, gaps, and future directions
Further studies are necessary to understand whether abnormal neurodevelopment is present across other brain regions and to what extent abnormal neurostructure is related to abnormal brain function, behavioral aspects of the disease, and alterations in cognitive, social and functional development. Essential questions that remain unanswered concern how these abnormalities predict clinical course or the social, academic, personal, and functional impairments present in PBD. Additionally, it is largely unknown which brain abnormalities represent risk factors, resilience factors, early disease markers, or some combination of these. Studies that integrate information from different imaging modalities and theoretical perspectives should help parse these effects. Longitudinal studies will be crucial to help to discern developmental and progressive neurophysiological aspects of PBD. Finally, future studies need to parse the independent and interactive effects of PBD in relation to treatment effects and response markers, as well as common comorbidities such as ADHD, anxiety, and substance use disorders.
9 PERIPHERAL BIOMARKERS
9.1 Established findings and consensus
Numerous studies over the past decade have examined peripheral biomarkers among adults with BD, focusing most consistently on a pattern of increased inflammatory and oxidative stress markers and decreased neurotrophic markers, particularly during acute mood episodes.235-238 Peripheral biomarkers, particularly those that fluctuate in relation to symptoms, have the potential for clinical application such as in the selection, prediction, and assessment of treatments. In contrast to the relatively broad literature on neuroimaging and neurocognition, there is a relative dearth of studies on peripheral biomarkers among youth with BD. However, in recent years there has been growing interest in this topic. Given the nascent literature, the following is a synopsis rather than a conclusive demonstration of replicated findings.
In a study of 30 adolescents with PBD, hypomanic symptoms were associated with levels of high-sensitivity c reactive protein (hsCRP). Brain-derived neurotrophic factor (BDNF) and interleukin 6 (IL-6) were negatively associated with each other, and cardiovascular high-risk levels of hsCRP were observed in 40% of the sample.239 A subsequent larger study on the topic based on 123 young adults in the Course and Outcome of Bipolar Youth study found that, controlling for comorbidity and treatment, tumor necrosis factor alpha (TNF-α) was associated with the proportion of time with psychosis, IL-6 was associated with the proportion of time with subthreshold mood symptoms and with any suicide attempt, and hsCRP was associated with maximum depression severity over the preceding 6 months.240
Two controlled studies of adolescents with PBD provide support for elevated inflammation. The first study (18 BD patients, 13 major depressive disorder [MDD] patients and 20 HCs) found significantly higher levels of nuclear factor kappa beta (NF-κβ) in peripheral blood mononuclear cells, monocytes, and lymphocytes, higher IL-1β plasma levels, and greater TNF-α-induced elevations in NF-κβ in adolescents with mood disorders than in HCs.241 The second study (40 BD patients and 20 HCs) found elevated IL-6 and TNF-α levels among adolescents with PBD.242
A recent study of 30 adolescents with BD found that oxidative stress markers were lower as compared to adults with BD, and lipid hydroperoxide (LPH) levels were associated with a proxy measure of atherosclerosis but not with mood symptoms or medications.243 A population-based study of young adults found that those with BPSD, but not those with MDD, had increased protein oxidative damage compared to HCs.244 A study of adolescents with or at risk for PBD found that adolescents with PBD had significantly lower LPH vs controls, with non-symptomatic adolescents at familial risk falling between the other groups.245 Finally, a study of 29 adolescents with PBD and 25 HCs found that LPH−BDNF correlations were significantly different between adolescents with PBD and HCs, and that, among adolescents with PBD in the top half of the distribution of BDNF levels, greater LPH levels were associated with poorer executive function.246
In an early biomarker study, platelet-derived BDNF protein and lymphocyte-derived BDNF mRNA levels were reduced among 26 medication-free youth with PBD compared to HCs; mRNA BDNF levels increased significantly in a subset of 19 participants after 8 weeks of treatment.247 A study of 30 adolescents with BD found a significant association between BDNF levels and amygdala volumes,248 whereas other findings from the same sample indicated no association between neurotrophic factors and hippocampal volumes.249
Similar to blood-based biomarkers, the literature regarding the genetics of PBD is sparse. A family-based association study found that genes coding for the serotonin transporter (solute carrier family 6 member 4), BDNF, and catechol-O-methyltransferase (COMT) were not significantly associated with PBD,250 whereas a prior study based on the same sample found that four of 28 single nucleotide polymorphisms (SNPs) in the dopamine transporter gene (SLC6A3) were associated with PBD, of which only rs40184 remained significant after correction for multiple comparisons.251 Another family-based association study found that two SNPs in early growth gene 3 (EGR3) were nominally significantly associated with PBD,252 as were glutamate decarboxylase 1 (GAD1)253 and BDNF val66 alleles.254 Most recently, a genetic study based on participants in the Treatment of Early Age Mania study found that a calcium channel, voltage-dependent, L type, alpha 1c subunit (CACNA1C) haplotype was associated with PBD. Whereas the CACNA1C rs1006737 SNP has been previously associated with adult BD in genome-wide association studies,255 it was the rs10848632 CACNA1C SNP that was nominally associated with PBD in SNP analyses.256
9.2 Limitations, gaps, and future directions
Despite the substantial increase in research findings in this area over the past 5 years, important limitations and gaps remain. Most studies have been constrained by small samples, limited covariate modeling, and cross-sectional design. Ultimately, the clinical application of peripheral biomarkers will require that findings are significant in the heterogeneous samples in which these biomarkers would be employed, as reflected by a variety of comorbidities, different subtypes of PBD, and varying medication regimens. Studies using repeated measures afford the advantage of within-subject analyses, which mitigate the impact of heterogeneity and state-related changes. With regard to genetics, larger studies are needed, as are studies examining the link between genetic markers and intermediate phenotypes, such as neuroimaging, in PBD. In addition to these considerations, studies that incorporate biomarkers as objective predictors, treatment targets, and/or correlates of treatment response are warranted.
10 CONCLUSIONS
There has been substantial progress in the area of PBD over the past 20 years. As data have accumulated and controversy has dissipated, the field has moved past existential questions about PBD toward defining and pursuing pressing clinical and scientific priorities that remain. The overall body of evidence supports the position that perceptions about marked international and developmental differences have been overstated, albeit that additional research on these topics is warranted. Traction toward improved outcomes will be supported by continued emphasis on phenomenology, prospective clinical epidemiology, pathophysiology, measurement-based quantitative approaches including digital phenotyping, and novel therapeutics.
Despite the substantial increase in consensus regarding phenomenology and epidemiology, there remain outstanding questions about the prevalence of PBD in pre-pubertal children. Whereas the existence of PBD in pre-pubertal children has long been recognized, and indeed described in Kraepelin's seminal text,257 reliable and valid estimates of the epidemiologic and clinical prevalence of PBD in pre-pubertal children are lacking. With the progress that has been made, in terms of parsing PBD from chronic irritability without episodic mania/hypomania, in terms of increased recognition of PBD, and in terms of screening and diagnostic instruments, the field is now better positioned to examine this topic than it was as recently as a decade ago.
In terms of pathophysiology, it is important to consider the context of the Research Domain Criteria (RDoC) initiative, which suggests the value of integrating dimensional liability traits alongside DSM-driven diagnoses and diagnostic criteria. In addition to all the inherent complexity of BD in adults, including differences across mood states, comorbidities, and BD subtypes, among others, research focusing on youth includes the added challenge of developmental differences. Future research should focus on age effects on the biology, manifestations, and treatment of PBD.
Future research should also focus on meaningfully integrating multiple methodologic levels of analysis. Thus far, there is a paucity of research that combines, for example, multiple neuroimaging approaches, neuroimaging and/or neurocognition with biomarkers, and/or neurobiology and clinical epidemiology. To the extent that the field can apply the large sample sizes and prospective measures of cohort studies toward improved understanding of the neurobiology of PBD, findings will become increasingly clinically and heuristically relevant.
From a treatment perspective, it will be important to begin addressing functional outcomes, rather than simply focusing on symptom reduction. Even during recovery, youth with PBD demonstrate impaired psychosocial functioning. Improved understanding of the factors underlying these impairments will allow for targeted preventive and treatment approaches. More studies with longer periods of acute treatment and maintenance studies will both help to clarify stabilization and relapse prevention. Representative epidemiologic, not just clinical, PBD samples show very high rates of suicidality and multi-comorbidity alongside very low rates of treatment, even though “over-treatment” and “over-diagnosis” garner more attention.258, 259 The high degree of complexity and severity of PBD, even in the relatively early years of the disease, is in part an outcome of delaying or avoiding treatment altogether. It is crucial that the clinical and scientific community continues collaborative efforts, together with consumers, families, schools, and other stakeholders, toward public education and stigma reduction.
Finally, prevention strategies must be an area of emphasis in the decade ahead. Findings suggest that some psychotherapies and perhaps nutritional interventions might be low-risk options for early stage/prodromal intervention. Here, prevention refers not only to the prevention of BD, which is an important but relatively distal goal, but also to the prevention of comorbidity accumulation (eg, substance use disorders), suicidality, treatment refractoriness, neurocognitive and functional impairment, and adverse physical health outcomes. Indeed, the physical implications of BD, particularly in terms of future cardiovascular disease, are increasingly recognized.79 Just as it is important for primary care providers to recognize the increased cardiovascular risk associated with PBD, it is important for our own field to begin integrating brain−body considerations in the assessment and treatment of PBD. The therapeutic and preventive potential of physical exercise for PBD, for example, is an almost entirely untouched topic.260, 261
While many questions remain unanswered, or insufficiently answered, we are hopeful that progress over the next decade will be far less constrained by controversies that, although critical in defining the directions of the field, also slowed progress and limited collaboration. At a time when clinically relevant psychiatric research faces the ironically dichotomous pressures of the RDoC era (ie, choosing between DSM and RDoC), it is important to retain focus on the importance of the categorical BD diagnosis in terms of treatment selection, clinical course, and familial risk. We are optimistic that liberally applied integration strategies (ie, dimensional as well as categorical views, neurobiology alongside clinical epidemiology, and pharmacologic alongside psychosocial interventions) will best serve the needs of our field and our constituents.
DISCLOSURES
Dr Birmaher receives funds for research from the National Institute of Mental Health. He receives royalties for publications from: Random House, Inc., Lippincott Williams & Wilkins, APA Press, and UpToDate. Dr. Carlson receives funds for research from the NIMH and Patient Centered Outcomes Research Institute (PCORI). Dr. Chang is an unpaid consultant for GSK, Lilly, and BMS. He is on the DSMB for Sunovion. In the past 3 years he has received research support from GSK and Merck, and has been a consultant for Actavis and Janssen. Dr. DelBello has received research support from NIMH, NIDDK, and PCORI, as well as Otsuka, Lundbeck, Sunovion, Pfizer, Johnson and Johnson, Supernus, Amarex, and Shire; and has received consulting/advisory board fees/honoraria from Pfizer, Lundbeck, Sunovion, Supernus, Takeda, Johnson and Johnson, Neuronetics, and Akili. Dr. Findling receives or has received research support from, acted as a consultant for and/or served on a speaker's bureau for Actavis, Akili, Alcobra, American Academy of Child & Adolescent Psychiatry, American Psychiatric Press, Bracket, CogCubed, Cognition Group, Coronado Biosciences, Elsevier, Epharma Solutions, Forest, Genentech, GlaxoSmithKline, Guilford Press, Ironshore, Johns Hopkins University Press, KemPharm, Lundbeck, Medgenics, Merck, NIH, Neurim, Novartis, Otsuka, PCORI, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Syneurx, Takeda, Teva, Tris, Validus, and WebMD. Dr. Fristad receives research funds from Janssen, publication royalties from Guilford Press, American Psychiatric Press and Child & Family Psychological Services, and honoraria from Physician's Post-Graduate Press. Dr. Goldstein has nothing to disclose. Dr. Hillegers has nothing to disclose. Dr. Kim has nothing to disclose. Dr. Kowatch is a consultant on data-safety monitoring boards for Forest and Pfizer; he is faculty for the REACH Institute. Dr. Miklowitz receives funds for research from the NIMH and royalties from Guilford Press and John Wiley & Sons. Dr. Nery's spouse is an employee of Eli Lilly & Company. Dr. Perez Algorta has no disclosures/conflicts of interest. Dr. Van Meter has no disclosures/conflicts of interest. Dr. Wozniak has nothing to disclose personally; her spouse has received speaker honoraria from Otsuka, royalties from UptoDate, consultation fees from Advance Medical, FlexPharma, and Merck; and research support from UCB Pharma, NeuroMetrix, and Luitpold. Dr. Youngstrom has consulted with Pearson, Otsuka, Lundbeck, Joe Startup Technologies, and Western Psychological Services about psychological assessment, and receives funding from NIMH.




