Initiation of Anticoagulants During the COVID-19 Pandemic in Sweden: An Interrupted Time Series Analysis
Funding: This work was performed as part of the Nordic COHERENCE project, project no. 105670 funded by NordForsk under the Nordic Council of Ministers and the EU-COVID-19 project, project no. 312707 funded by the Norwegian Research Council's COVID-19 Emergency Call and by a grant from the Novo Nordisk Foundation to the University of Copenhagen (NNF15SA0018404). The SCIFI-PEARL project which supplies the data for the Swedish part of this analysis has basic funding based on grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (Avtal om Läkarutbildning och Forskning/Medical Training and Research Agreement) grants ALFGBG-938453, ALFGBG-971130, ALFGBG-978954 and previously from a joint grant from FORTE (Swedish Research Council for Health, Working Life and Welfare) and FORMAS (Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning), grant 2020-02828. P-JS was funded by an individual grant from the Regional Health Authority of Northern Norway (Helse Nord; HNF1648-22).
ABSTRACT
The COVID-19 pandemic may have increased anticoagulant initiation due to the thrombogenic nature of the disease or decreased due to the societal impact of the pandemic. We aimed to study the effect of the COVID-19 pandemic on initiation of anticoagulants in Sweden. We conducted a single interrupted time series analysis on the monthly cumulative incidence of nonvitamin K antagonist oral anticoagulants (NOAC), warfarin, or heparins, before and after March 2020, using SCIFI-PEARL dataset. For anticoagulants in total, there were no statistically significant changes or differences in the trends of initiation after the start of the pandemic. There was a slight numerical decrease in initiation after the onset of the pandemic, particularly for NOACs. For individuals aged ≥ 65 years, however, the immediate decrease in initiation was considerable for NOACs. The prepandemic declining trend of warfarin initiation seemed to attenuate, that is, became less negative, after March 2020. We did not find any profound effect of the COVID-19 pandemic on the initiation of anticoagulants in total. However, among individuals aged ≥ 65 years, a notable immediate decrease in initiation of NOACs was observed. Furthermore, the onset of the pandemic may have attenuated the downward temporal trend in initiation of warfarin use.
Plain English Summary
The COVID-19 pandemic had many societal impacts. In this registry-based study with data from the entire Swedish population, we studied the impact of the pandemic on the use of drugs that prevent blood clots (anticoagulant drugs). The main finding was that the start of the pandemic did not have a large impact on the use of such drugs. But we found that there were some changes in which type of blood clot-preventing drugs were used right after the pandemic started, including less use of warfarin.
1 Introduction and Background
Venous thromboembolism (VTE) is common in hospitalised COVID-19 patients; around one-third of COVID-19 patients in the intensive care unit (ICU) had VTE in the early pandemic, while in non-ICU inpatients it was closer to 5% [1]. The incidence of VTE was especially high during the first wave of the COVID-19 pandemic [1, 2]. The first wave in Sweden occurred between March and September in 2020, with a peak in ICU patients and deaths early in April [3, 4]. The risk of VTE remains particularly elevated for the first weeks after COVID-19 diagnosis, up to 6 months for pulmonary embolism [2].
Since early in the pandemic, all hospitalised COVID-19 patients are generally given prophylactic anticoagulant treatment, if not contraindicated, usually in the form of low-molecular weight heparins (LMWH). Prophylactic anticoagulant treatment is, however, rarely needed after discharge or in the outpatient setting [1, 5]. Established VTE on the other hand is normally treated with therapeutic doses of heparins in inpatient care, with possible transition to oral anticoagulants (OACs) such as nonvitamin K antagonist oral anticoagulants (NOACs) or warfarin to be continued in the outpatient setting. The treatment duration for OACs is usually at least 3 months [1]. In this regard, an increase in the prescribing and dispensing of OACs during the COVID-19 pandemic could be expected.
However, the pandemic may also have had a negative impact on the use of anticoagulants due to the reduced access to health services, for example, visits to general practioners, specialist clinics or pharmacies. During the early phases of the pandemic, there was a decline in visits to healthcare, including reduction in weekly visits to primary care physicians, for example, a 9% relative reduction in weekly visits in Sweden [6], and reduced number of hospital admissions, even for clinical events such as myocardial infarction [3] or stroke [7]. Thus, the pandemic may have led to reduced or postponed initiation of anticoagulants, particularly OACs.
Given these possible opposing trends in anticoagulant initiation, we aimed to study the effect of the COVID-19 pandemic on new dispensing of anticoagulant drugs, that is, OACs or heparins, in Sweden.
2 Materials and Methods
This was a registry-based before-after-study using single interrupted time series analysis (SITSA). In this study, we utilise monthly prescription dispensing data to assess the incidence of initiating anticoagulant drugs before and after the start of the COVID-19 pandemic in Sweden in March 2020.
The study population consisted of all individuals ≥ 18 years resident in Sweden on 1 March 2019 and who were dispensed an anticoagulant drug between 1 March 2019 and 31 July 31, 2022 and had no dispensings of anticoagulant drugs in the 14 months preceding the study period, that is, 1 January 2018 to 28 February 2019—a fixed washout window. The study participants were thus ‘new on anticoagulants’ as a group [8]. The study period covers the four waves of COVID-19 in Sweden, which, despite slight differences in definition [2, 8], correspond to Wave 1 (March to September 2020), Wave 2 (October 2020 to January 2021), Wave 3 (February to June 2021) and Wave 4 (July 2021 to March 2022). [4]
Data were derived from the ‘Swedish COVID-19 Investigation for Future Insights—a Population Epidemiology Approach using Register Linkage (SCIFI-PEARL)’ cohort dataset, described in detail elsewhere [9]. In summary, SCIFI-PEARL consists of a record-linkage database, with the aim to study the impact and consequences of COVID-19 in Sweden [9]. In SCIFI-PEARL, a range of Swedish national health and population registries, such as the national database of notifiable diseases (SmiNet) [10], the National Patient Register [11], National Prescribed Drug Register [12], and the Cause of Death Register [13], is linked for individuals based on the Swedish national personal identification numbers [14]. The data include information on confirmed COVID-19 cases and many clinical and sociodemographic variables. For the analysis of initiation of anticoagulants in this study, we used data originating from the National Prescribed Drug Register, covering data on all dispensed prescriptions for the entire Swedish population, including dates of prescription and dispensing, ATC-codes (according to the anatomical therapeutic chemical classification system) [15] and dispensed amounts [12].Drugs administered in hospitals are not captured. Other variables as stated below were derived from the National Patient Register and Statistics Sweden; see reference for details about the registries [9].
We defined anticoagulant drugs based on the registered ATC codes of the dispensed drugs and divided them into three groups: (1) NOACs (B01AF* or B01AE*), (2) warfarin (B01AA03) or (3) heparins (B01AB*). Total use of anticoagulants was defined as dispensing from either of those three groups. Platelet inhibitors or other antithrombotic drugs were thus not considered in this study.
The outcome measure was the monthly cumulative incidence of patients initiating an anticoagulant drug, expressed as per hundred thousand inhabitants. The patients were counted in the numerator of the month when they got their first anticoagulant dispensing, that is, counted only once within each subanalysis; for total use of anticoagulant drugs that would mean the first dispensing from any of the three anticoagulant groups, while for the individual groups the first dispensing of that particular subtype of anticoagulants was considered. For the monthly denominator, we used the population (≥ 18 years) of Sweden at the beginning of the month in question, derived from Statistics Sweden [16], with patients who died, emigrated, or that already had experienced the outcome subtracted. Thus, we had cumulative incidences for each separate month of the 41-month study period.
Descriptive variables of the study population include previous drug use, defined as dispensing of various drug groups (ATC third level) in the 26 months preceding the pandemic (1 Jan 2018 to 28 Feb 2020), comorbidities based on ICD-10 codes in the 5 years preceding the pandemic (1 Jan 2015 to 28 Feb 2020), age at first dispensing, sex, marital status (married, not married), educational level (≤ 9 years, 9–12 years, > 12 years), occupational status (employed, unemployed/retired) and country of birth (Sweden, Nordic countries but Sweden, EU28 without Nordic, other European countries, out of EU28, with EU28 referring to the 28 member countries of the European Union).
We conducted SITSA of the monthly cumulative incidence using March 2020 as the time of the interruption, March 2019 to February 2020 as the pre-interruption period and April 2020 to July 2022 as the postinterruption period. We fitted linear segmented regression models [17], with a lag of 12 months to account for autocorrelation, and predicted the trends before and after the interruption based on the data (SAS or Stata macros included in references) [18-20]. We verified our time series data for normality of the distribution of residuals by the Shapiro–Wilk test (p = 0.08), stationarity by the augmented Dickey-Fuller test (p = < 0.0001) and the presence of no substantial autocorrelation through visual inspection of autocorrelation plots, partial autocorrelation plots and plots of residuals.
The results were visualised by SITSA graphs of observed values and predicted trends, for anticoagulants in total and were stratified on anticoagulant group, age (18–64 years, ≥ 65 years) and sex. Data management and analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Graphs were created in Stata 17.1 (Stata Corp, College Station, TX, USA).
The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies [21].
3 Results
The study population consisted of 492 177 new users of anticoagulant drugs, with 55% females and a mean age of 63.3 (± 17.8) years at first dispensing (Table 1). Most of the patients received heparins (n = 252 672, 51%) as their first anticoagulant dispensing, followed by NOACs (n = 232 500, 47%) or warfarin (n = 7005, 1.4%). Notably, over one-third of the study population had been dispensed drugs from ≥ 15 other drug groups (ATC 3rd level, Table 1). Eleven percent (n = 55 359) had a previous diagnosis of atrial fibrillation or atrial flutter (ICD-10 I489).
| Variables | n | % |
|---|---|---|
| All | 492 177 | 100 |
| Sex | ||
| Male | 223 214 | 45.4 |
| Female | 268 963 | 54.6 |
| Age at first dispensing | ||
| 18–39 | 69 028 | 14.0 |
| 40–64 | 155 515 | 31.6 |
| 65–84 | 221 951 | 45.1 |
| ≥ 85 | 45 683 | 9.3 |
| Marital statusa | ||
| Married | 235 632 | 48.1 |
| Not married | 254 746 | 52.0 |
| Missing | 1799 | |
| Educational levela | ||
| ≤ 9 years | 113 440 | 23.5 |
| 9–12 years | 212 511 | 43.9 |
| > 12 years | 157 856 | 32.6 |
| Missing | 8370 | |
| Occupational statusa | ||
| Employed | 262 646 | 53.6 |
| Unemployed/retired | 227 731 | 46.4 |
| Missing | 1800 | |
| Country of birth | ||
| Sweden | 407 746 | 82.9 |
| Other Nordic countries Sweden | 19 852 | 4.0 |
| EU28 without Nordic countries | 16 279 | 3.3 |
| Other European countries | 12 166 | 2.5 |
| Outside Europe | 36 107 | 7.3 |
| Missing | 27 | |
|
No. of dispensed drug groups other than anticoagulantsb |
||
| 1–4 | 52 039 | 10.6 |
| 5–9 | 131 476 | 26.7 |
| 10–14 | 135 763 | 27.6 |
| ≥ 15 | 172 899 | 35.1 |
| Most common dispensed drugs other than anticoagulantsb, c | ||
| Paracetamol (N02B) | 349 351 | 71.0 |
| Opioids (N02A) | 288 668 | 58.7 |
| Drugs for constipation (A06A) | 253 286 | 51.5 |
| Penicillins (J01C) | 248 342 | 50.5 |
| Drugs for peptic ulcer and GERD (A02B) | 232 114 | 47.2 |
| NSAIDs (M01A) | 219 195 | 44.5 |
| Beta blockers (C07A) | 199 664 | 40.6 |
| Lipid modifying drugs (C10A) | 166 080 | 33.7 |
| Calcium channel blockers (C08C) | 145 988 | 29.7 |
| Hypnotics and sedatives (N05C) | 143 052 | 29.1 |
- a Up to January 2020.
- b ATC third level codes between 1 Jan 2018 and 28 Feb 2020.
- c Ten most frequent drug groups.
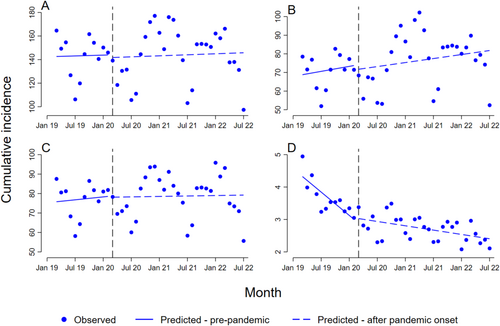
For anticoagulants in total, there was no substantial or statistically significant changes or differences in the trends of initiation of anticoagulants after the start of the COVID-19 pandemic (Figure 1A and Table 2).
| Drug group | Coefficient | 95% CI | p |
|---|---|---|---|
| Anticoagulants in total | |||
| b0: Prelevel | 143 | 127–158 | < 0.0001 |
| b1: Pretrend | 0.12 | −1.8–2.0 | 0.91 |
| b2: Postlevel change | −2.3 | −29–25 | 0.87 |
| b3: Posttrend change | 0.028 | −1.8–1.8 | 0.98 |
| b1 + b3: Posttrend | 0.14 | −1.0–1.3 | 0.80 |
| NOAC | |||
| b0: Prelevel | 68 | 62–75 | < 0.0001 |
| b1: Pretrend | 0.45 | −0.39-1.3 | 0.30 |
| b2: Postlevel change | −2.3 | −18.3-13.6 | 0.77 |
| b3: Posttrend change | −0.088 | −0.98-0.80 | 0.85 |
| b1 + b3: Posttrend | 0.36 | −0.35–1.1 | 0.32 |
| Heparins | |||
| b0: Prelevel | 76 | 67–84 | < 0.0001 |
| b1: Pretrend | 0.25 | −0.82–1.3 | 0.65 |
| b2: Postlevel change | −0.43 | −13–12 | 0.95 |
| b3: Posttrend change | −0.21 | −1.2–0.79 | 0.68 |
| b1 + b3: Posttrend | 0.038 | −0.47-0.54 | 0.88 |
| Warfarin | |||
| b0: Prelevel | 4.4 | 4.1–4.7 | < 0.0001 |
| b1: Pretrend | −0.12 | −0.16–0.079 | < 0.0001 |
| b2: Postlevel change | 0.043 | −0.24–0.32 | 0.77 |
| b3: Posttrend change | 0.098 | 0.058–0.14 | < 0.0001 |
| b1 + b3: Posttrend | −0.022 | −0.029--0.015 | < 0.0001 |
The monthly modelled predicted prepandemic cumulative incidence was 143 per 100 000 (b0), with a slight immediate numerical decrease in the cumulative incidence of 2.3 per 100 000 at the interruption point in March 2020 (b2). There was no profound difference, neither visually or statistically, between the monthly trend after the onset of the pandemic and the prepandemic trend (b3 = 0.028, 95% CI: 1.8–1.8).
The NOACs showed a small numerical but not statistically significant immediate postinterruption level decrease in the cumulative incidence (Figure 1B; b2 = −2.3, 95% CI: 18.3–13.6). The trend after the onset of the pandemic for the NOACs was quite similar to the positive prepandemic trend (b3 = −0.088, 95% CI: 0.98–0.80), while the postpandemic trend after the onset of the pandemic for the heparins was noticeably more level after March 2020 (Figure 1C; b3 = −0.21, 95% CI: 1.2–0.79).
Warfarin showed by far the lowest prepandemic cumulative incidence (b0 = 4.4) and was the only group where statistically significant changes were detected (Table 2, Figure 1D). Warfarin exhibited an opposite trend compared to the NOACs and the heparins. A prepandemic negative trend (b1 = −0.12, 95% CI: 0.16–0.079) became marked less negative after the start of the pandemic (b3 = 0.098, 95% CI 0.058–0.14).
In the sex-stratified analyses, an immediate numerical decrease (postlevel change) was found among females for anticoagulants in total (b2 = −10, 95% CI: 39–19), NOACs (b2 = −6.5, 95% CI: 20–7.3) and heparins (b2 = −4.3, 95% CI: 21–12) (Table S1 and Figure S1), while an immediate numerical increase was found among males for the same groups (b2 = 5.4, 95% CI: 20–31, b2 = 1.8 95% CI: 16–20, and b2 = 3.4, 95% CI: 5.1–12, respectively) (Table S2 and Figure S2). There were no noticeable sex differences for warfarin.
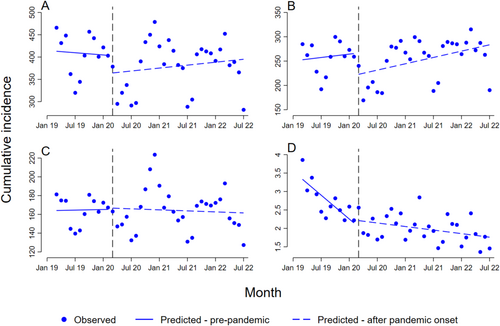
When the analysis was restricted to individuals ≥ 65 years (Figure 2 and Table 3), the most noteworthy finding was that anticoagulants in total and the NOACs showed a considerable immediate decrease (b2 = −41, 95% CI: 105–24 and b2 = −45, 95% CI: 86–3.4, respectively), followed by positive trends after the onset of the pandemic; statistically significant for the NOAC group. There were no profound changes in the trends for the heparins. Warfarin among individuals ≥ 65 years followed the same trends as in the total population.
| Drug group | Coefficient | 95% CI | p |
|---|---|---|---|
| Anticoagulants in total | |||
| b0: Prelevel | 414 | 375–452 | < 0.0001 |
| b1: Pretrend | −0.83 | −5.7-4.1 | 0.74 |
| b2: Postlevel change | −41 | −105-24 | 0.22 |
| b3: Posttrend change | 1.9 | −2.6-6.4 | 0.41 |
| b1 + b3: Posttrend | 1.1 | −1.4-3.6 | 0.39 |
| NOAC | |||
| b0: Prelevel | 252 | 228–275 | < 0.0001 |
| b1: Pretrend | 1.2 | −1.7–4.1 | 0.44 |
| b2: POSTLEVEL change | −45 | −86–3.4 | 0.04 |
| b3: posttrend change | 1.0 | −1.8–3.8 | 0.49 |
| b1 + b3: Posttrend | 2.2 | 0.4–3.9 | 0.01 |
| Heparins | |||
| b0: Prelevel | 164 | 150–178 | < 0.0001 |
| b1: Pretrend | 0.11 | −1.7–1.9 | 0.91 |
| b2: Postlevel change | 1.5 | −23–26 | 0.90 |
| b3: Posttrend change | −0.29 | −1.9–1.3 | 0.73 |
| b1 + b3: Posttrend | −0.18 | −1.2–0.80 | 0.72 |
| Warfarin | |||
| b0: Prelevel | 3.4 | 3.2–3.7 | < 0.0001 |
| b1: Pretrend | −0.11 | −0.14–0.070 | < 0.0001 |
| b2: Postlevel change | 0.080 | −0.22–0.38 | 0.60 |
| b3: Posttrend change | 0.092 | 0.056–0.13 | < 0.0001 |
| b1 + b3: Posttrend | −0.016 | −0.025–0.0076 | < 0.001 |
Among individuals aged 18–64 years, as opposed to individuals ≥ 65 years, there was a numerical immediate increase for anticoagulants in total and for the NOAC group (b2 = 9.7, 95% CI: 15–34, and b2 = 11, 95% CI: 2.1–24, respectively) (Table S3 and Figure S3).
4 Discussion
In this nationwide registry-based study, we found no profound long-term change in the monthly cumulative incidence of initiation of anticoagulants in total (NOACs, warfarin, or heparins combined) after March 2020. There was a slight numerical drop in the cumulative incidence immediately after the onset of the pandemic, particularly for the NOACs. For individuals aged ≥ 65 years; however, the immediate decrease in incidence at the pandemic onset was considerable, especially for the NOACs. The declining trend of warfarin after the onset of the pandemic seemed to attenuate, that is, became less negative, after March 2020.
The impact of the COVID-19 pandemic on antithrombotic drugs has been explored in previous studies utilising the interrupted time series design, in populations from the United States, Italy and the United Kingdom [22-28]. The analyses vary in included antithrombotic drugs, outcome measure and data sources—from hospital consumption statistics [27], prescription volumes [22, 24, 25], to individual-level data [23, 26, 28]—making direct comparisons challenging. Most similar to the current study is the studies by Antonazzo et al. and Yang et al. where the authors explored the effect of the onset of the pandemic on the incidence of initiating OACs in the Tuscany region of Italy [23] or among atrial fibrillation patients in the USA [28], respectively. They found somewhat similar results as in the present study: an immediate decrease in the incidence of NOACs followed by a positive trend during the first pandemic period. Antonazzo et al. report an immediate drop in the incidence for vitamin K antagonists [23], while Yang et al. report an immediate increase for warfarin [28]. However, both studies are in agreement with our findings of a more levelled out trend, that is, a less negative trend, for warfarin in the first part of the pandemic [23, 28]. In the US hospitals, the start of the pandemic led to an immediate decrease in the total sales of antithrombotic drugs, and OACs and parenteral antithrombotics in particular, with a return to, or even above, prepandemic levels [27]. Warfarin showed a negative trend both through the prepandemic period and the pandemic period, with a numerical immediate increase in the monthly units. [27] Results from aggregated prescription data from England showed an immediate increase for all antithrombotics (ATC: B01) in general [22] and OACs in particular [22], in contrast with our findings. Other UK data, using weekly prescription counts, indicated no immediate change for NOACs or warfarin after the first lockdown in March, 2020 [25]; the positive prepandemic trend of NOAC became less positive during the pandemic, while a stable prepandemic trend for warfarin became negative, at least in the early phase of the pandemic [25]. In summary, studies have reported either an immediate increase or immediate drop in the utilisation of anticoagulants, with a later normalisation. Our finding of a less negative trend for warfarin after the onset of the pandemic is, however, similar to the findings of Antonazzo et al. [23] and Yang et al. [28] but seemingly more contrary to the findings of Dale et al. [25]
In Sweden, the COVID-19 strategy relied more on voluntary actions of individuals following official guidance rather than government-imposed restrictions or mandatory measures, compared to many other countries [3, 6]. A cross-national comparative study showed that the start of the pandemic did not seem to lead to profound changes in the overall drug consumption in Sweden, except for some indications of stockpiling in the first month(s); the prescription volume, expressed as DDD/TID, decreased by 0.8% for antithrombotic drugs (ATC: B01) in the first year of the COVID-19 period (March 2020–February 2021) compared to the preceding year [29]. Similar findings of stockpiling before the March 2020 lockdown, including for anticoagulants, have been reported from the UK [25]. The US data also indicate that the start of the pandemic led to increased possession of OACs, that is, fewer OAC users had a 7-day gap in the treatment and more patients had OACs in excess (‘15-day excess’) in analyses of drug adherence [26]. Nevertheless, the disruptive societal effects of the COVID-19 pandemic may still have affected the initiation of and adherence to anticoagulant treatment negatively. For example, another study shows that the time in therapeutic range for warfarin patients decreased after the onset of the pandemic, probably due to reduced monitoring [30]. Furthermore, the pandemic may have negatively influenced adherence among patients with a pre-existing poor adherence to NOACs [31]. Results from a Swedish study indeed show that the use of anticoagulants had a protective effect on COVID-19-associated hospitalisation and death among atrial fibrillation patients, spurring the authors to call for action to improve adherence in this patient group [8].
Our results indicate that the prepandemic negative trend in warfarin initiation levelled out after the start of the pandemic, that is, a less negative trend, in accordance with previous studies [23, 28]. The explanation for this relative increase in incidence of use outside of hospital above expectations from prepandemic trends is not entirely clear. NOACs are the first-line agent for new-onset atrial fibrillation [32] and outpatient treatment of deep vein thrombosis or pulmonary embolism [33, 34], including COVID-19 patients with confirmed VTE [11]. There is, however, more than 50 years of clinical experience with warfarin [34], while the NOACs are still relatively new. COVID-19 is in some cases associated with an increase in antiphospholipid antibodies, although not quite antiphospholipid antibody syndrome [1]. Warfarin is generally preferred over NOACs among patients with antiphospholipid antibody syndrome in need of anticoagulation [34]. Due to such concerns, one could assume that the ‘tried and trusted’ warfarin might be preferred among some clinicians and during the exceptional period of the COVID-19 pandemic. In contrast to an increase for warfarin, a switch from warfarin to NOACs was recommended by some authors, due to the reduced ability to monitor the warfarin treatment during the pandemic, among other factors [35]. In lack of a clear explanation, our finding regarding warfarin should be carefully interpreted, but similar findings from other populations suggest that this is not a chance finding.
A major strength of this study is a complete national population as source for the data, with highly valid dispensing data including prescriptions from all providers in primary- and secondary care, and complete follow-up. Furthermore, the SITSA design, based on an interruption that can be seen as a natural experiment, can provide evidence of causal effects with relatively simple means [20].
The study has a few weaknesses. The SITSA methodology assumes that there is no time-dependent confounding, and Linden, in a key ITSA paper, highlights ‘the need for caution with these methods if there are multiple policy shifts in the time window around the implementation of the intervention’ and in the case of pre-existing trends [20]. Many policy shifts happened rapidly at the beginning of the pandemic [3], and NOACs showed a pre-existing positive temporal trend, while warfarin a negative temporal trend [36]. We could not adjust the group-level aggregated data used in the SITSA at an individual level; we stratified on age and sex groups but were unable to calculate the incidence for other groups due to the lack of external information for the denominators. Furthermore, we only had 12 time points before the interruption, which limited our ability to account for seasonality in dispensing, e.g., around holidays. Data on in-hospital treatment with anticoagulants, for example, unfractionated heparin, are unfortunately not available in Sweden. Treatment of COVID-19 associated VTE will thus only be captured in our study for patients who were continued on either heparins or OACs after discharge. The risk of VTE was the highest in the first wave of the pandemic. [2] However, in the current analysis, we did not make a distinction between the different waves or strains of the coronavirus. This may have hidden or obscured effects occurring for instance only during the first wave. Since we focused on the timing of the start of the pandemic rather than individual confirmed COVID-19 cases, we are unable to disentangle the pathophysiological effects of the disease from the societal changes during the pandemic.
We did not find a profound effect of the COVID-19 pandemic on the incidence of initiating anticoagulants overall, other than a slight numerical decrease in the incidence immediately after the pandemic onset in March 2020. However, among individuals aged ≥ 65 years, a considerable immediate decrease in incidence of initiating NOACs was observed. Furthermore, the onset of the pandemic may have attenuated the downward temporal trend in the incidence of warfarin initiation. The interpretation of the findings is challenging since the societal impact on anticoagulant dispensing, that is, possible decline in the incidence of initiating anticoagulants, may be opposed by the thrombogenic nature of COVID-19 itself, i.e., possible increased incidence of initiating anticoagulants.
Author Contributions
P.-J.S. contributed to the analysis, created the graphs, wrote the draft and the final manuscript. M.H., B.W. and F.N. wrote the protocol and designed the study. M.H. conducted the data management and statistical analyses. All authors aided in the interpretation of the results and critically revised the manuscript. All authors have approved the final manuscript.
Acknowledgements
We acknowledge the initial work of former master student in pharmacy Shiva Farrokhipour, conducted at Uppsala University (supervised by MH and BW). We also acknowledge Professor Jonas Oldgren, Uppsala Clinical Research Centre and Department of Medical Sciences at Uppsala University, for comments on an earlier version of the manuscript.
Ethics Statement
This study was approved by the Swedish Ethical Review Authority (SCIFI-PEARL, 2020–01800 with subsequent amendments) and is reported in accordance with the RECORD-PE statement [37].
Conflicts of Interest
MHH and PS have no relevant financial or non-financial interests to disclose. FN owns some AstraZeneca shares, received study grand from ALF and FORTE/FORMAS and Unrestricted grant to institution for PASS study from BAYER/OXON. BW is Board member European Drug Utilization Research group and Scientific advisor to the Swedish Regulatory Agency.
Open Research
Data Availability Statement
Data are available upon reasonable request from the corresponding author.




