Histological and histochemical study of the digestive system of the Argentine anchovy larvae (Engraulis anchoita) at different developmental stages of their ontogenetic development
Abstract
In this work, histological and histochemical features of the larval digestive system of Argentine anchovy Engraulis anchoita were described. Structural changes during ontogenetic development were also characterized, and comparisons between the beginning and the end of larval development were made. Histological sections of larvae were subjected to histochemical and routine histological techniques to localize and differentiate glycoproteins (GPs). Both at an early and a late larval stage, the oesophageal goblet cells reacted more intensely than those of the rest of the digestive tract, and only the oesophagus exhibited metachromasia with toluidine blue techniques at different pHs, thus revealing diverse GPs at different concentrations. The GPs histochemical composition in the intestine varied with the developmental stage and the intestinal zone. The absence of goblet cells characterized the foregut; however, they started differentiation at an advanced stage in the midgut. These cells could be detected in the hindgut both at the beginning and at the end of development. The attached glands showed a varied glycoprotein composition. The digestive tract of E. anchoita presented a high level of complexity, related to the multiple functions of mucus in the digestive tract, such as lubrication, protection, antimicrobial function and ionic and osmotic regulation.
Introduction
Most of the organ systems of marine teleost fishes that spawn pelagic eggs originate either during the late embryonic period or during the vitelline larval stage, becoming functional at the end of this stage. Maturation of the organ systems must involve functional changes related to modifications of the larval ecology (O'Connell 1981).
In fish larvae, the successful development of the digestive system is crucial for survival and growth, because it allows capture, ingestion, digestion and absorption of food. Although first-feeding larvae could be morphologically able to capture food, the digestive system needs a series of changes during the ontogenetic development before being completely functional (Canino and Bailey 1995; Chen et al. 2006).
The adult Argentine anchovy Engraulis anchoita is a carnivore that feeds on small- to medium-sized planktonic crustaceans and eventually on phytoplankton; larvae are almost exclusively zooplanktophage, chiefly feeding on eggs and copepod nauplii (Ciechomski 1965; Sato et al. 2011). Its distribution goes from Cabo Frio, Brazil (23ºS), to the southern end of Golfo San Jorge (47°S). There are two adult populations: Patagonic (41°–47°S) that reproduces in summer and the one from Buenos Aires (north of 41°S) that reproduces in spring (Sánchez and Ciechomski 1995). This marine species, of great bioeconomic value, is the main dietary component of various commercial species, such as hakes, mackerels, weakfishes and marine mammals and birds. In view of the current intensive exploitation of fishery resources, the anchovy represents an option of economic potential. Particularly, the northern population, object of study of this work, is in terms of biomass, the greatest potential fishing resource of the south-western Atlantic.
Diaz et al. (2011a,b) made a spatio-temporal analysis of the nutritional state of anchovy larvae and its relation to hydrographical features and food availability and initiated a general histological characterization of larvae of the species. The histological analysis of the digestive system of anchovy larvae, particularly of the liver and the digestive tract, represents a sensible index of nutritional condition and can be employed to estimate the inanition incidence in larval mortality along the ontogenetic development. On the other hand, larvae at an early developmental stage are more susceptible to nutritional stress than those at an advanced stage, probably due to a less availability of reserve substances.
Sieg (1998) proposed the use of a histological method for classifying the E. anchoita larval phases and defined six developmental stages (DS) based on yolk presence, oesophageal epithelium height, folding of the different digestive tube portions, the degree of development of the swim bladder and pancreas and ventral jaw. The fish intestinal tube exhibits a morphological and functional diversity, according to feeding habits and body shape (Ray and Moitra 1982; Buddington et al. 1997). A common feature in the teleostean digestive tract is mucus, with glycoproteins (GPs) as its most important component (Díaz et al. 2008). Fiertak and Kilarski (2002) have proposed that the GP composition of the digestive tract depends on the species and the intestinal region, but not on the food type ingested. These mucins develop many functions such as lubrication, protection against proteolytic and antimicrobial degradation, and ionic and osmotic regulation (Díaz et al. 2002, 2003, 2006; Domeneghini et al. 2005; Yashpal et al. 2007). Murray et al. (1994) suggest that the posterior oesophageal GPs of some fish could be involved in the pregastric digestion. Also, the epithelial surfaces and their secreted GPs play a fundamental role in mediating the relationship between environment and organism (Mittal et al. 2002; Domeneghini et al. 2005).
The aim of this work was to analyse the GP composition and distribution in the digestive system of larvae of E. anchoita from the Buenos Aires population. Comparisons between the beginning and the end of larval development were made to evaluate GP distribution variations and its functional significance during the ontogeny of larvae.
Materials and Methods
Animals
Larvae employed in this study were obtained from the OB-06/11 campaign carried out by the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP). Larval developmental stage was defined according to Sieg (1998). Only Sieg's stages III, IV and VI were used because comparisons were made between the beginning and the end of larval development. The most conspicuous difference related to digestive system according to Sieg (1998) between studied stages was the degree of gut epithelium folding. Stage III is characterized by an unfolded gut epithelium, and stage IV, by an unfolded hindgut epithelium but certain degree of ventral midgut epithelium folding; and in contrast, midgut and hindgut epithelia of developmental stage VI are completely folded.
To estimate larval DS, the standard length (SL) was measured as the distance between snout and notochord ends. DS was later histologically verified. Larvae of 4–6 mm SL corresponded to Sieg's stage III and the beginning of stage IV and were herein grouped as “early” developed larvae. On the other hand, 10- to 12-mm-SL larvae corresponded to stage VI and were considered into an “advanced” developmental stage (Diaz et al. 2011a).
Sampling
The larvae were separated in situ, fixed in pH7 formol buffer during 2 h and then conserved in 70% alcohol. Samples were routinely processed and embedded in paraplast. Longitudinal 4-μm-thick histological serial sections were cut with a microtome. The obtained sections were processed according to the standard protocol and then treated with routine techniques: haematoxylin and eosin (H/E) and Masson's trichromic for general morphology and tissue differentiation studies.
Traditional histochemistry
Histological techniques used for GPs identification are presented in Table 1. Sections were stained with (i) PAS (periodic acid–Schiff's reagent) to demonstrate periodate reactive vicinal diols; (ii) α-amylase digestion before PAS reaction for a control of GPs presence with oxidizable vicinal diols; and (iii) KOH/PA*S (saponification-selective periodic acid–Schiff reaction) to allow the characterization of total sialic acid. The saponification with 0.5% potassium hydroxide in 70% ethanol for 30 min at room temperature was performed to deacetylate sialic acid residues and was followed by PA*S; (iv) PA*/Bh/KOH/PAS (periodic acid–borohydride reduction – saponification – periodic acid–Schiff reaction): this method was carried out using a 2-h oxidation at room temperature with 1% periodic acid. The aldehydes generated by the initial oxidation were reduced to Schiff-unreactive primary alcohols with sodium borohydride (PA-Bh). Following saponification (KOH), only sialic acids with O-acyl substituents at C7, C8 or C9 (or which had two or three side chains O-acyl substituents) and O-acyl sugars were PAS-positive; (v) KOH/PA*/Bh/PAS (saponification-selective periodic acid–borohydride reduction – periodic acid–Schiff reaction) for neutral sugar characterization; (vi) AB pH 2.5 (Alcian Blue 8GX pH 2.5, Mallinckrodt Chemical Works, St. Louis, MO, USA) to demonstrate GPs with carboxyl groups (sialic acid or uronic acid) and/or with O-sulphate esters; (vii) AB pH 1.0 (Alcian Blue 8GX pH 1.0) to demonstrate GPs with O-sulphate esters; (viii) AB pH 0.5 (Alcian Blue 8GX pH 0.5) to demonstrate highly sulphated GPs; (ix) AB pH 2.5/PAS (Alcian Blue 8GX pH 2.5/periodic acid–Schiff staining) to demonstrate GPs with carboxyl groups and GPs with O-sulphate esters (turquoise), periodate reactive vicinal diols (magenta), and GPs presence with carboxyl groups and with GPs with O-sulphate esters together with periodate reactive vicinal diols (purple); (x) TB pH 5.6 (toluidine blue) to demonstrate GPs with O-sulphate esters and carboxyl groups; (xi) TB pH 4.2 (toluidine blue) to demonstrate GPs with O-sulphate esters.
| Procedure | Dyeing interpretation | References |
|---|---|---|
| PAS | GPs with oxidizable vicinal diols and/or glycogen | Mc Manus (1948) |
| KOH/PA*S | GPs with sialic acid residues | Culling et al. (1976) |
| KOH/PA*/Bh/PAS | GPs with oxidizable vicinal diols and O-acyl sugars | Volz et al. (1987a,b) |
| PA/Bh/KOH/PAS | Sialic acid residues with O-acyl substitution at 7C, 8C or 9C, and O-acyl sugars | Reid et al. (1973) |
| AB pH 2.5/PAS | Acid GPs and GPs with oxidizable vicinal diols | Mowry (1963) |
| AB pH 2.5 | GPs with carboxyl groups and O-sulphate esters | Lev and Spicer (1964) |
| AB pH 1.0 | GPs with O-sulphated esters | Lev and Spicer (1964) |
| AB pH 0.5 | Highly sulphated GPs | Lev and Spicer (1964) |
| TB pH 5.6 | GPs with O-sulphate esters and carboxyl groups | Lison (1953) |
| TB pH 4.2 | GPs with O-sulphate esters | Lison (1953) |
- PAS, periodic acid/Schiff; PA*S, periodic acid/Schiff at low temperature and pH (oxidation with 0.4 mM periodic acid in 1.0 M hydrochloric acid at 4 °C); PA, periodic acid; KOH, saponification; Bh, borohydride; AB, Alcian Blue; TB, toluidine blue; GPs, glycoproteins.
Evaluation of labelling intensities was based on subjective estimates of the authors through the examination of two sections per sample of all the animals tested.
Results
Histological characterization
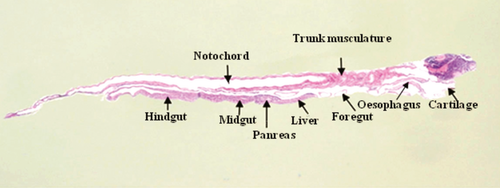
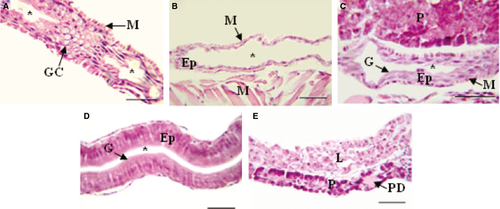
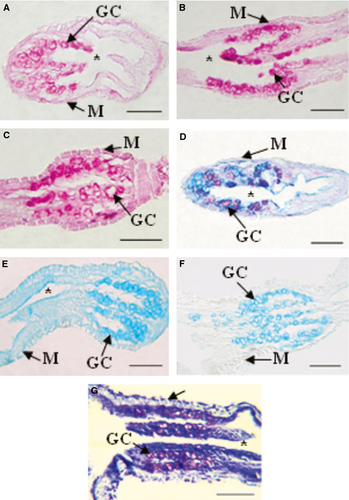
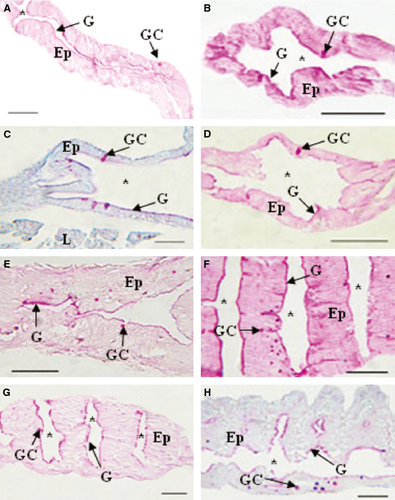
The digestive tube of larvae was divided into four sections: oesophagus, foregut, midgut and hindgut (Figs 1 and 2). The oesophagus of early developed larvae was covered by a slightly stratified epithelium with numerous goblet cells (Fig. 3A); the foregut had a low simple cubic epithelium (Fig. 3B), the absorptive cells of the midgut were high cubic with noticeable basal nuclei and evident microvilli towards the light of the intestine, thus forming the striated layer of this organ (Fig. 3C), while in the hindgut, villi began to form and the epithelium had dominant cylindrical cells (Fig. 3D); the well-developed liver and pancreas were located in the medial portion of the larvae; the liver cells were cuboidal, with a voluminous nucleus; the pancreas cells had a basal nucleus with very eosinophilic zymogen granules in the apical portion (Fig. 3E); pancreatic ducts of small calibre were also observed, and the endocrine portion of the gland consisted of a single Langerhans islet. More developed larvae showed a greater number of stratified epithelium layers in the oesophagus (Fig. 4A); the foregut was still straight with a simple cuboidal epithelium and a conspicuous brush border (Fig. 4B); the midgut began pleating and the hindgut clearly distinguished with abundant villi covered by a simple cylindrical epithelium with goblet cells and an evident brush border (Fig. 4C,D); the accessory glands were larger (Fig. 4E,F).

Histochemical characterization
The implementation of different histochemical techniques to demonstrate the presence of GPs in the digestive system of the Argentine anchovy, E. anchoita, at an early and late larval development is showed on Tables 2 and 3, respectively.
| Dyeing | Oesophagus | Foregut | Midgut | Hindgut | L | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epit Cell | MC | Ent | MC | Bb | Ent | MC | Bb | Ent | MC | Bb | |||
| PAS | 1 | 2 | 1 | – | 1 | 2 | – | 2 | 2 | 2 | 2 | 2 | 2 |
| KOH/PA*S | 1 | 0 | 1 | – | 1 | 1 | – | 1 | 1 | 0 | 1 | 1 | 1 |
| KOH/PA*/Bh/PAS | 1 | 2 | 1 | – | 2 | 2 | – | 2 | 2 | 0–3a | 3 | 2 | 1 |
| PA/Bh/KOH/PAS | 1 | 2 | 1 | – | 2 | 1 | – | 2 | 1 | 0–3a | 2 | 2 | 2 |
| AB pH 2.5 | 1 | 3 | 1 | – | 2 | 1 | – | 3 | 1 | 0–2a | 2 | 1 | 1 |
| AB pH 1.0 | 1 | 3 | 1 | – | 2 | 1 | – | 2 | 1 | 0–2a | 1 | 1 | 1 |
| AB pH 0.5 | 1 | 3 | 1 | – | 2 | 1 | – | 2 | 1 | 0–2a | 1 | 0 | 0 |
| AB pH 2.5/PAS | 1 | 3 | 1PAS | – | 3AB | 1 | – | 2AB | 1 | 1AB | 3PAS | 2 | 2 |
| AT pH 5.6 | 2 | 3m | 2 | – | 2 | 3 | – | 3 | 3 | 0–3a | 3 | 3 | 3 |
| AT pH 4.2 | 2 | 3m | 2 | – | 2 | 3 | – | 3 | 3 | 0–3a | 3 | 3 | 3 |
- Epit Cell, epithelial cells; Ent, enterocytes; MC, mucous cells; Bb, brush border; L, liver; P, pancreas; 0, negative reaction; 1, weak positive reaction; 2, moderate positive reaction; 3, strong positive reaction.
- a Two goblet cell types were differentiated.
| Dyeing | Oesophagus | Foregut | Midgut | Hindgut | L | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epit Cell | MC | Ent | MC | Bb | Ent | MC | Bb | Ent | MC | Bb | |||
| PAS | 1 | 2 | 1 | – | 2 | 1 | 2 | 2 | 1 | 0–3 | 2 | 2 | 1 |
| KOH/PA*S | 1 | 0 | 1 | – | 1 | 1 | 0 | 1 | 1 | 0–3 | 1 | 1 | 1 |
| KOH/PA*/Bh/PAS | 2 | 2 | 1 | – | 1 | 2 | 0 | 2 | 2 | 0–3a | 2 | 2 | 2 |
| PA/Bh/KOH/PAS | 2 | 3 | 2 | – | 2 | 2 | 0 | 2 | 2 | 0–3a | 2 | 2 | 2 |
| AB pH 2.5 | 2 | 3 | 2 | – | 2 | 2 | 0–2a | 2 | 2 | 0–3a | 2 | 2 | 2 |
| AB pH 1.0 | 1 | 2 | 0 | – | 1 | 0 | 0–2a | 1 | 1 | 0–3a | 1 | 1 | 1 |
| AB pH 0.5 | 0 | 2 | 0 | – | 1 | 0 | 0–2a | 0 | 1 | 0–3a | 1 | 0 | 0 |
| AB pH 2.5/PAS | 1 | 3AB–PASa | 2 | – | 2AB | 1 | 3PAS | 2PAS | 2 | 3PAS–ABa | 3PAS | 2 | 2 |
| AT pH 5.6 | 2 | 3m | 2 | – | 2 | 3 | 0–3a | 3 | 3 | 0–3a | 3 | 3 | 3 |
| AT pH 4.2 | 2 | 3m | 2 | – | 2 | 3 | 0–3a | 3 | 3 | 0–3a | 3 | 3 | 3 |
- Epit Cell, epithelial cells; Ent, enterocytes; MC, mucous cells; Bb, brush border; L, liver; P, pancreas; 0, negative reaction; 1, weak positive reaction; 2, moderate positive reaction; 3, strong positive reaction.
- a Two goblet cell types were differentiated.
Early developmental stage
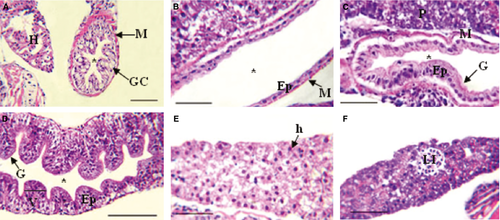
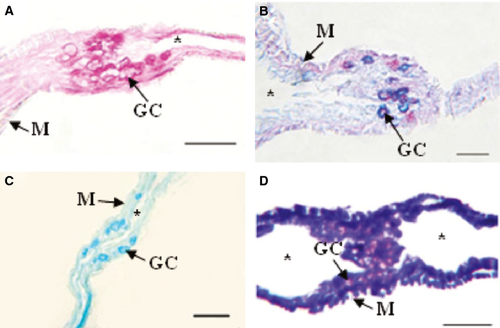
The epithelial cells that covered the oesophageal mucosa and the foregut reacted weakly to the PAS, KOH/PA*/Bh/PAS, KOH/PA*S and PA/Bh/KOH/PAS techniques, evidencing GPs with oxidizable vicinal diols, neutral GPs and GPs with sialic acid residues with and without substitution at C7, C8 and/or C9, and O-acyl sugars, respectively. Further, with AB at different pHs, small amounts of sulphated GPs were detected. Enterocytes of the midgut and hindgut reacted moderately to PAS indicating moderate amounts of GPs with oxidizable vicinal diols, and the reaction with PA/Bh/KOH/PA*S techniques evidenced GPs with oxidizable vicinal diols and O-acyl sugars. The KOH/PA*S and PA/Bh/KOH/PA*S techniques showed GPs with sialic residues with and without O-acyl substitution at C7, C8 and/or C9, and O-acyl sugars. The reaction with AB at different pHs evidenced sulphated GPs. Goblet cells were mainly observed in the oesophagus and less so in the hindgut. The majority of the utilized techniques indicated that the oesophageal goblet cells reacted more intensely than those of the rest of the digestive tube, showing metachromasia with TB at both pHs (Fig. 5D). In particular, the histochemical techniques employed revealed high concentrations of GPs with oxidizable vicinal diols, GPs with sialic acid residues with substitutions at C7, C8 and/or C9, O-acyl sugars and sulphated GPs (Fig. 5A,C). With the combined AB/PAS techniques, groups of AB- and PAS-positive goblet cells could be differentiated (Fig. 5B). In the hindgut, these cells reacted with the KOH/PA*/Bh/PAS (Fig. 7B) and PA/Bh/KOH/PAS techniques, revealing neutral GPs and GPs with sialic acid residues with O-acyl substitutions at C7, C8 and/or C9, and O-acyl sugars. With the AB techniques at different pHs, carboxylated and sulphated GPs were identified. The positive reaction with the PAS technique suggested the presence of GPs with oxidizable vicinal diols (Fig. 7A). The combined AB/PAS technique allowed the identification of goblet cell groups slightly positive to AB in the hindgut, revealing small quantities of carboxylated and sulphated GPs, and also the glycocalyx of the foregut and midgut exhibited this type of GPs, while GPs with oxidizable vicinal diols (PAS-positive) predominated in the glycocalyx of the hindgut. The liver and the pancreas weakly reacted to the KOH/PA*S and AB techniques at different pHs, thus suggesting small amounts of GPs with sialic acid residues and sulphated groups. The technique KOH/PA*/Bh/PAS evidenced moderate amounts of neutral GPs in the liver while scarce in the pancreas. After a ptyalin enzymatic pretreatment, the PAS technique revealed small amounts of GPs with oxidizable vicinal diols and glycogen. The moderate PA-/Bh-/KOH-/PAS-positive reaction unveiled GPs with sialic acid residues substituted at C7, C8 and/or C9, and O-acyl. The AT orthochromatic reaction at both pHs demonstrated moderate to high amounts of carboxylated groups.
Advanced developmental stage
The epithelial cells of the whole digestive tract reacted weakly to PAS and KOH/PA*S, thus indicating the presence of small quantities of GPs with oxidizable vicinal diols and sialic acid residues, and reacted moderately to PA/Bh/KOH/PAS, evidencing moderate amounts of GPs with sialic acids residues with O-acyl substituents at C7 and C8 and with O-acyl sugars. The AB techniques at different pHs revealed GPs with carboxylated and sulphated groups, with proportions according to the digestive tract section. Goblet cells predominated in the oesophagus and occurred moderately in the hindgut. This cell type was first observed in the midgut. As in the early stage, the oesophageal goblet cells reacted more intensely than those in the digestive tube, and only the latter presented AT metachromasia at both pHs (Fig. 6G), showing high GPs concentrations with oxidizable vicinal diols, GPs with sialic acid residues and GPs with sulphate groups. The few goblet cells of the midgut reacted moderately to the PAS technique, evidencing GPs with oxidizable vicinal diols, and the AB technique at different pHs identified GPs with very sulphated groups. The positive reaction of this cells with PA/Bh/KOH/PAS and KOH/PA/BH/PAS techniques suggests the presence of GPs with sialic acid residues substituted at O-acyl at C7, C8 and/or C9, O-acyl sugars and neutral GPs (Fig. 7D,E). These same cells reacted intensely in the hindgut with the PAS technique, revealing large quantities of GPs with oxidizable vicinal diols with KOH/PA*/Bh/PAS, revealing neutral GPs (Fig. 7F), and with the PA/Bh/KOH/PAS technique, evidencing GPs with sialic acid residues substituted at O-acyl at C7, C8 and/or C9, and O-acyl sugars (Fig. 7G). The weakly positive reaction with KOH/PA*S evidenced GPs with sialic acid residues. The combined technique AB/PAS unveiled different goblet cell types according to sections of the digestive tract: cells secreting GPs with oxidizable vicinal diols (PAS-positive), cells producing carboxylated and sulphated (AB-positive) GPs and cells with mixed secretions (Fig. 7H). Further, this combined technique showed predominance of acid GPs in the glycocalyx of the foregut and of GPs with oxidizable vicinal diols in the midgut and hindgut. The histochemical reaction of the liver and the pancreas was similar in both glands. The PAS reaction after a ptyalin digestion revealed small amounts of GPs with oxidizable vicinal diols and glycogen. The KOH/PA*S reaction showed a slightly positive reaction, indicating the presence of scarce amounts of GPs with sialic acid residues. The AB techniques at different pHs evidenced small quantities of carboxylated and sulphated GPs. Reactions with KOH/PA*/Bh/PAS and PA/Bh/KOH/PAS revealed moderate amounts of neutral GPs with oxidizable vicinal diols and sialic acid residues with O-acyl substituted at C7, C8 and/or C9, and O-acyl sugars.
Discussion
This study is the first histochemical description of the digestive system of the Argentine anchovy larvae, E. anchoita.
The digestive tract general morphology proved to be similar to the one described for other teleosts (Santamaría et al. 2004; Kozarić et al. 2008). Both developmental E. anchoita larval stages analysed presented the oesophageal mucosa lined by a stratified squamous epithelium with abundant goblet cells. Adults of this species have shown the oesophagus divided in two zones, one of which possesses an epithelium similar to the one reported in this work (Díaz et al. 2003).
The foregut was straight and lined by a simple cuboidal epithelium with microvilli, becoming columnar in the midgut; villi outlined the hindgut. In later stages, these villi became more developed and the brush borders thickened, thus suggesting an increase in the surface area for absorption and digestion. The enterocytes, which are involved in absorption, were the most abundant cells of the intestine. This intestine morphology coincides with the description of other teleosts (Santamaría et al. 2004; Kozarić et al. 2008).
The histochemical analysis showed that goblet cells of E. anchoita larvae firstly appeared in the oesophagus and later in the intestine, as found in species like Gadus morhua (Morrison 1993), Sparus aurata L. (Elbal et al. 2004), Pagrus pagrus (Darias et al. 2007) and Diplodus puntazzo (Micale et al. 2008).
Both the differentiation of goblet cells and their histochemical characteristics vary according to the species. Observations showed that goblet cells of E. anchoita differentiated at the beginning of larval development. This is in accordance with observations in Solea solea (Boulhic and Gabaudan 1992), Sander lucioperca (Ostaszewska 2005), Silurus glanis (Kozarić et al. 2008) and other species (Baglole et al. 1997; Gisbert et al. 1999; Ribeiro et al. 1999; Green and McCormick 2001; Gisbert and Doroshov 2003; Mai et al. 2005; Alvarez-González et al. 2008), where oesophageal goblet cells develop concurrently with exogenous feeding. However, in Scophthalmus maximus (Segner et al. 1994), Sparus aurata (Sarasquete et al. 1995), Diplodus sargus (Ortiz-Delgado et al. 2003), Pagellus erythrinus (Micale et al. 2006) and other species (Morrison 1993; Hamlin et al. 2000; García-Hernández et al. 2001; Gisbert et al. 2004; Darias et al. 2005; Faulk et al. 2007; Hachero-Cruzado et al. 2009), goblet cells arise at later developmental stages. The size and the amount of these cells increase as larvae develop into juveniles.
Goblet cells secrete different types of mucosubstances that differ in their histochemical characteristics. In this work, we observed variations in mucin subtypes in goblet cells, recognizing a neutral GP-secreting group, sulphated GPs and GPs with sialic acid residues group. These GPs combined secretion were also found in juveniles of Sparus aurata (Domeneghini et al. 1998), Acipenser baeri (Gisbert et al. 1999), Silurus glanis (Kozarić et al. 2008) and Claris batrachus (Raji and Norouzi 2010). Each of the GP types has been associated with a specific functional role. Thus, the combination of different kinds of GPs with different functions can provide an efficient activity control, in the oesophagus in this case. Because, unlike superior vertebrates, fish have no salivary glands, it is possible that the mucus secreted by the oesophageal goblet cells plays a lubrication and protection function of the whole alimentary canal mucosa (Scocco et al. 1998). Glycoproteins with sialic acid residues prevent viral and bacterial infections, avoiding viruses to recognize their receptors and stopping the attack of bacterial sialidase (Zimmer et al. 1992). Moreover, the combined secretion of GPs has been described by several authors as an ionic and osmotic regulator mechanism (Domeneghini et al. 1998; Sarasquete et al. 2001). Likewise, neutral GPs cooperate in the digestion of food and its transformation into chyme, as well as in the absorption of simple substances, such as disaccharides and short-chained fatty acids (Sarasquete et al. 2001).
In the developmental stages here studied, we did not observe the presence of a stomach. In a morphological and glandular sense, the anchovy larvae do not show a differentiated stomach until they reach a very advanced maturity stage (Ciechomski 1967; Diaz et al. 2011a). As in other species, the appearance of this organ occurs between 33 and 50 mm TL in relation to the transition towards a juvenile stage (Tanaka 1973).
The enterocytes that line the fish intestine have in their apical surface a GP layer called the glycocalyx that protects them from proteolytic enzymes (Ross and Pawlina 2007). In this work, we observed that in both stages, the foregut of the E. anchoita larvae possesses a glycocalyx with predominance of acid GPs, while in the hindgut, neutral GPs predominate. However, larvae at an early stage of development have the midgut covered by a glycocalyx composed of acid GPs, and as larvae develop, they are replaced by neutral GPs.
Some species, like Barbus conchonius, have intestinal goblet cells before the first exogenous feeding (Rombout et al. 1978); others, like Dicentrarchus labrax, undergo a differentiation process at more advanced stages (García-Hernández et al. 2001; Zambonino-Infante et al. 2008). Goblet cells of E. anchoita larvae begin to proliferate in the hindgut, being absent in the foregut and midgut during the early stages, and they secrete a mixture of neutral and acid GPs. In posterior larval developmental stages, they begin to appear in the midgut. Glycoproteins produced by the intestinal goblet cells could be useful for faeces lubrication, whereas those in other intestinal regions protect the intestinal mucosa and facilitate the nutrients absorption (Domeneghini et al. 1998). As in other teleosts (Sinha 1976; Ribeiro et al. 1999; Elbal et al. 2004), the amount of goblet cells in the digestive tract of E. anchoita increased with larval development, and again, agreeing with other species (Raji and Norouzi 2010), its distribution increased towards the distal portion of the intestine. Besides, another source of GP secretion at both larval stages was the epithelial cells. Both the scarce goblet cells and the epithelial cells secreted GPs with oxidizable vicinal diols, GPs with sialic acid residues with or without O-acyl substitutions at C7, C8 and/or C9, O-acyl sugars and acid GPs. However, goblet cells differed from epithelial cells because each GP class is elaborated in greater concentration.
The accessory glands, liver and pancreas, are present from the beginning of development. In some species, like Dicologoglossa cuneata, these organs develop, while the larva feeds on the endogenous reserves, being rapidly functional after hatching (Hoehne-Reitan and Kjørsvik 2004; Herrera et al. 2010). The presence of zymogen granules in the exocrine pancreas, observed in the studied E. anchoita stages, would support this fact. However, the PAS and α-amylase/PAS techniques revealed a low liver glycogen reserve. Diverse authors propose that the glycogen stored in fish liver can noticeably diminish due either to swimming activity (Miller et al. 1959; Pritchard et al. 1971) or to malnutrition (Love 1974). Moreover, there are variations in glycogen content during the feeding–starvation cycle (O'Connell and Paloma 1981). The larvae here studied belong to a night sampling, that is, they were in a pre-ingest phase; therefore, the glycogen stored during the day should have already been consumed. For this reason, closeness to the moment of food intake, which occurs during the first hours of the day (Viñas and Ramírez 1996), seems to be the best explanation to the low glycogen levels found in this work.
In conclusion, this study has permitted to evidence that from the beginning of their ontogeny, the E. anchoita larvae possess the morphological equipment necessary to successfully accomplish the digestive and absorptive processes. That is, they have structures such as enterocytes and microvilli, secretory goblet cells with a great variety of GPs, pancreatic cells with little zymogen particles and ducts connecting the accessory glands to the intestine. That is to say, the digestive system of the Argentine anchovy is functional at its very early developmental stage, increasing its complexity and size along the larval period.
The resulting information constitutes a contribution to the knowledge of the biology of E. anchoita, a species that has an enormous fishery potentiality due to its abundance and to the situation of overexploitation of most of the traditional fishery resources. Recently, the fishery certification of the Buenos Aires anchovy population (, Federal Fisheries Council) was materialized; this fact poses the need to deepen the knowledge of the biology of this species all along its ontogeny.
Acknowledgements
This study was supported by a Grant from Universidad Nacional de Mar del Plata, Buenos Aires, Argentina, and the INIDEP. We wish to thank BIP Oca Balda personnel for their help during sample collection and special thanks to Marta Estrada for her help during histological procedures. This is INIDEP contribution N° 1797.




