Understorey plant community and light availability in conifer plantations and natural hardwood forests in Taiwan
Abstract
Questions
What are the effects of replacing mixed species natural forests with Cryptomeria japonica plantations on understorey plant functional and species diversity? What is the role of the understorey light environment in determining understorey diversity and community in the two types of forest?
Location
Subtropical northeast Taiwan.
Methods
We examined light environments using hemispherical photography, and diversity and composition of understorey plants of a 35-yr C. japonica plantation and an adjacent natural hardwood forest.
Results
Understorey plant species richness was similar in the two forests, but the communities were different; only 18 of the 91 recorded understorey plant species occurred in both forests. Relative abundance of plants among different functional groups differed between the two forests. Relative numbers of shade-tolerant and shade-intolerant seedling individuals were also different between the two forest types with only one shade-intolerant seedling in the plantation compared to 23 seedlings belonging to two species in the natural forest. In the natural forest 11 species of tree seedling were found, while in the plantation only five were found, and the seedling density was only one third of that in the natural forest. Across plots in both forests, understorey plant richness and diversity were negatively correlated with direct sunlight but not indirect sunlight, possibly because direct light plays a more important role in understorey plant growth.
Conclusions
We report lower species and functional diversity and higher light availability in a natural hardwood forest than an adjacent 30-yr C. japonica plantation, possibly due to the increased dominance of shade-intolerant species associated with higher light availability. To maintain plant diversity, management efforts must be made to prevent localized losses of shade-adapted understorey plants.
Nomenclature
-
- Boufford et al. (2003)
-
Introduction
Forest canopies are light-absorbing objects that efficiently reduce light availability in the forest understorey (Kimmins 1996), resulting in low light availability for understory plant growth. In most forests there is high variability of understorey light availability, which allows the co-existence of plants with different light requirements (Canham et al. 1990). Therefore, changes in light environments have profound effects on productivity and diversity of understorey vegetation (Menges 1985; Carol et al. 1992; Zipperlen & Press 1996; Williams et al. 1999; Myers et al. 2000). Changes in forest canopy structure can substantially affect understorey light environments, so management practices leading to changes in canopy cover have major impacts on understorey light environments and plant diversity.
Forest plantations are expanding at the expense of natural forests in many regions, such as South America (Nahuelhua et al. 2012) and East Asia (Wang et al. 2009, 2010; Meyfroldt & Lambin 2012). Some argue that forest plantations may provide key ecosystem services and effectively sequester atmospheric carbon (Fang et al. 2001; Paquette & Messier 2010). Yet, others have observed that forest plantations do not typically maintain soil fertility levels seen in natural forests, and recommended avoiding the replacement of natural forests with plantations to maintain ecosystem sustainability (Halpern & Spies 1995; Liao et al. 2012). Converting natural forests to forest plantations not only affects key ecosystem processes such as soil respiration, nutrient cycling and carbon sequestration (Hyvönen et al. 2007; Wang et al. 2009; Sheng et al. 2010) but also affects canopy and understorey plant diversity (Ito et al. 2004; Nagaike et al. 2005; Kodani 2006).
Taiwan is a subtropical island (36 000 km2) located in Southeast Asia. Forests cover 58.5% (TFB 1995) of the land area of Taiwan and host most of the island's biodiversity. Economic pressures led to aggressive timber exploitation in low- to mid-elevation forests through the 1970s. Some harvested forest area was set aside for natural secondary succession, while other areas were converted to plantations. Cryptomeria japonica (L. f.) D. Don is the most widely planted species, covering 40 000 ha. It is native to Japan and was first introduced to Taiwan in 1906 (TFB 1997). However, due to changing market conditions, many privately owned conifer plantations have been abandoned, allowing natural succession to take place (TFB 1995). This provides a unique opportunity to study succession processes of abandoned plantations.
Most forest plantations in Taiwan are located in low- to mid-elevation areas (300–1500 m) where the natural vegetation is evergreen hardwood forest with relatively high diversity of canopy species. Currently we know very little about the consequences of such management history on secondary succession as it relates to tree and understorey plant diversity. Will diverse hardwood forests come back naturally through succession as those seen in old-field succession in North America (Pickett et al. 1987)? Over what time frame? Will secondary forests be missing some of the components or functional groups of the mature forest communities, especially those habitats with a long history of intensive utilization as seen in Puerto Rico (Chinea 2002)? Abandonment of C. japonica is not unique to Taiwan; in Japan, large areas planted with C. japonica were abandoned due to declining market value (Kodani 2006). A study of community development after the cessation of management in Kamikawa, central Japan, suggests that species richness and composition of the abandoned plantations will not return to primary forest conditions (Nagaike et al. 2005). In contrast, a study of structure and dynamics of tree populations on unsuccessful C. japonica plantations near the Shirakami Mountains indicates that through secondary succession, tree communities in the plantations are expected to become dominated by late successional species, although it will be a long process (Masaki et al. 2004). Thus, the stand development following the cessation of management can be diverse and site-dependent.
Community development following the abandonment of forest plantations could be very different in the plantation species’ native and introduced areas. Both in Kamikawa and Shirakami Mountains of Japan the native vegetation was temperate hardwood forest dominated by one to a few canopy species (Masaki et al. 2004; Nagaike et al. 2005), whereas in the areas of Taiwan where C. japonica was planted the natural vegetation is diverse subtropical to warm-temperate hardwood forests without any particularly dominant species. Introduction of C. japonica for plantations is not limited to Taiwan, it is widely planted in China (Da et al. 2009) and also common in Europe (Alian et al. 2006). Yet, compared to the detailed studies in Japan, we know very little about community development following the abandonment of C. japonica in its introduced areas. Understanding community development following the abandonment of forest plantations of non-native tree species is critical for sustainable forest management in the tropics and subtropics, at a time in which governments and populations recognize the importance of managing for a variety of ecosystem services, from provisioning to carbon storage, erosion control, non-native species prevention and biodiversity conservation. The trajectory of successional changes in the community following cessation of management of non-native forest plantations could vary among tropical vs temperate climates, island vs continental floras, across land forms and soil types, as well as with the intensity and extensiveness of plantation management. Abandoned plantations are an important part of the landscape in many places, and more empirical studies are needed before generalizations can be inferred. Documenting the relationships between light environment and plant diversity and composition in the understorey is a critical step in better understanding the succession of abandoned and unmanaged forest plantations.
Study objectives
We examined soil properties, light environments and plant diversity under natural hardwood forests and nearby abandoned C. japonica plantations in northeastern Taiwan to evaluate the consequences of widespread replacement of natural hardwood forests by C. japonica plantations on critical biotic and abiotic environmental factors. Because higher tree species diversity would lead to more diverse microhabitats, we hypothesized that the monoculture C. japonica plantation would have lower understorey plant species diversity than the mixed-species natural hardwood forest (H1). Because of the differences in expected plant diversity, we expected low similarity in understorey plant communities between the two forest types (H2). Because of the differences in microhabitats, we hypothesized that the relative abundance in the number of species and number of individuals of different functional groups (tree, shrub, grass, forb, woody vine, herbaceous vine and fern) are different between the two forest types (H3). Because of differences in the shape and distribution of tree crowns between the two forest types, we would expect that understorey light availability is different between the two forest types (H4). Finally, because light availability plays a key role in understorey plant growth and survival, we would expect it to be closely related to understorey plant diversity (H5).
Methods
Study site
The study was conducted at Fushan Experimental Forest and adjacent 35-yr-old C. japonica plantations in northeastern Taiwan. Annual precipitation ranges from 2900 to 6000 mm, and mean annual temperature is 18 °C, with the lowest monthly mean temperatures in January (12 °C) and highest in July (24 °C). Mean annual relative humidity is 96%; the forest is foggy throughout the year and rain occurs on more than 220 d annually, on average (Hsia & Hwong 1999; Lin et al. 2011).
Fushan Experimental Forest is characterized as a moist subtropical Lauro-Fagaceae forest without an observable dormant season. The forest ranges in elevation from 600 to 1200 m. The soil is characterized as very acidic (pH 3.8–5.0) with low bulk density (0.5–0.9 Mg·m−3) and very low base saturation (2–5%) (Horng & Chang 1996; Liang et al. 1997). There are 515 plant species belonging to 329 genera and 124 families within Fushan Experimental Forest (TFRI 1989). The dominant tree species are Castanopsis carlesii var. sessilis Nakai, Machilus thunbergii (Sieb. et Zucc.) Kostermans, Engelhardtia raxburghiana Wall., Meliosma squamulata Hance, Litsea acuminata (Blume) Kurata, Diospyros morrisiana Hance, Helicia formosana Hemsl and Pyrenaria shinkoensis (Hayata) Keng (Wang et al. 2000). The forest is multistoried, with scattered tree ferns (Alsophila podophylla Hook) and dense shrubs (mainly Blastus cochinchinensis Lour, H. formosana and Lasianthus obliquinervis Uerr.) and herbaceous cover. Total leaf area index (LAI) of the forest varies from 6 to 8, with canopy trees having a LAI of 3 to 6 (Lin et al. 1994). The forest has a mean tree canopy height of 9.4 ± 0.54 (±1 SE) m, mean DBH of 40.7 ± 3.0 cm, and tree density of 5630 ± 94 trees·ha−1 (Chen 2000).
The abandoned C. japonica plantations (400 ha total) are located 5 km southeast of the Fushan Experimental Forest, in an area of small hills surrounding two connected wetlands and several small agricultural fields. The elevation varies from 500 to 800 m. According to our interview with the older residents near the forest, the area was covered by natural hardwood forests and first cleared for farming ca. 1900. Different parts of the area have varied land-use histories. We limited our experimental set-up to areas with well-understood land-use history. We chose areas that were natural hardwood forests cleared for growing sweet potatoes ca. 110 yrs ago. During that period, the sweet potato fields were left fallow every 2–3 yrs, allowing soil fertility to recover. In the 1950s, C. japonica plantations replaced the sweet potato fields. In the 1970s the plantations were cut and second-generation C. japonica was planted. During the first 6 yrs, mechanical weed control was conducted three to four times per year. Then, due to low market value, management activities ceased in the 1980s. As a result, some hardwood species have invaded the plantations. C. japonica in the area has a mean height of 12.4 ± 0.39 m, DBH of 52.9 ± 6.9 cm and tree density of 6730 ± 49 trees·ha−1 (Chen 2000).
Field set-up
For both the natural hardwood forest and the C. japonica plantation, we selected six stands that are at least 50 m away from each other. To minimize topographic influences, all selected sites are on southeast slopes, but due to the complex topography, choosing sites with exactly identical slope and aspect was not possible. One 10 m × 10 m plot was set up in each stand to survey understorey light environments and plant richness/diversity.
Understorey plant diversity
We established three 10-m line transects in each of the six plots of each forest type, 2.5 m away from the edge of the plot and 2.5 m apart from each other. All plants with height less than 130 cm that intersected the line transects were recorded and identified to species with nomenclature following Boufford et al. (2003).
 (1)
(1)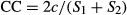 (2)
(2) (3)
(3)We classified understorey plants into seven functional groups: tree, shrub, grass, forb, woody vine, non-woody vine and fern. From the functional identities we estimated α functional diversity based on the Shannon index. Tree seedlings were further classified into shade-tolerant and shade-intolerant following reports of seedling photosynthetic capacity (Kohyama & Grubb 1994; Kuo & Fan 2003; Chu & Sheu 2005; Liou et al. 2006; Kuo & Lai 2008; Chen et al. 2013; Kuo 2013; Kuo et al. 2013; Tang et al. 2013; Kuo & Yang 2014). Due to the binary classification and that most of the seedling species have high shade tolerance, a species that is moderately shade-tolerant to shade-intolerant was classified as shade-intolerant.
Understorey light environment
Understorey light levels were assessed using hemispherical photography (Anderson 1964; Becker et al. 1988; Rich 1990). Hemispherical photographs were acquired from each plot at 2.5-m intervals at 1.3 m above the ground along the three transect lines used for the understorey plant survey, resulting in nine photographs for each plot and 54 photographs for each forest type. A previous study indicated that a minimum spacing of 2.5 m is required to avoid spatial autocorrelation of understorey light availability in the studied forests (Lin et al. 2003) and 1.3 m is just above the majority of ground vegetation (mostly herbaceous plants). The photographs were taken in February 2011, when LAI was close to its 16-yr mean, based on our long-term monitoring at Fushan Experimental Forest (Lin et al. 2011).
Hemispherical photographs were taken under uniform overcast sky conditions to maximize the contrast between openings and foliage. We used a five-megapixel Nikon Coolpix 4500 digital camera (Nikon, Tokyo, Japan) equipped with a Nikon FC-E8 fisheye lens (with a combined focal length equivalent to 7.2 mm and a combined F number of f/2.4). During photograph acquisition the camera was compass-oriented so that magnetic north was located at the top of the photographs, to allow the overlay of sun tracks for the calculation of direct radiation transmission (Rich 1990). Photographs were digitized, and then analysed using HemiView (Delta-T 2000) to determine availability of direct and indirect radiation relative to a location with no obstructions (Rich 1990; Lin & Chiang 2002; Lin et al. 2003).
Soil properties
Soil samples were taken at 0–15 cm, 15–30 cm and 30–60 cm depths from two randomly selected locations in each plot. Soil pH was measured with electrodes in a 1:1 soil/water suspension. Organic carbon was determined with a modified Walkley-Black procedure. Exchangeable K, Na, Ca, Mg and CEC were extracted using 1 M ammonium acetate (pH 7.0). The concentrations of K, Na, Ca, Mg and Al were measured using inductively coupled plasma mass spectrometry (ICP, Jobin-Yvon Horiba group, Jy2000; Edison, NJ, US). Kjeldahl N was extracted after incubation at 40 °C for 2 wk, with 2 M KCl. Available P was measured with the Bray No. 1 procedure. All soil chemical analyses were carried out in duplicate and the final determinations were obtained by averaging these values.
In situ gas exchange
We took foliar gas exchange measurements of the three most common understorey species in each of the two types of forest using a LI-6400XT Portable Photosynthesis System (Li-Cor Bioscience, Lincoln, NE, US). Within each plot, gas exchange measurements were taken on one newly mature leaf of each of six plants for each of the three most common species of each forest type. We measured gas exchange rate at 0, 20, 50, 100, 200, 400, 600, 800, 1000, 1500 and 2000 μmol·m−2·s−1 photosynthetic photon flux density (PPFD; 90% red, 10% blue diodes). During gas exchange measurements, the environmental conditions inside the cuvette were maintained at 25 °C block temperature (leaf and air temperatures were within 1 °C of block temperature), 1.0 kPa vapor pressure deficit, and 370 μl·L−1 CO2 concentration. Fan speed inside the cuvette was fast, and air flow rates were 500 μmol·s−1. Data for each measurement were collected only after the Sample and Reference infrared gas analysers were electronically equalized (‘Match’ function), and the measured data were stable (CVtot% ≤ 0.2). Gas exchange parameters were measured three times for each leaf, and averaged first by plant and then by species for each forest type.
Data analysis
We examined differences in light availability both among stands of each forest type and between the two forest types using nested ANOVA, with forest type as a fixed factor and stands within each forest type as a nested factor. We used two-way ANOVA to examine differences in soil properties, both between forest types and among depths. The relationship between understorey diversity, both species richness and exponential of Shannon entropy (for both species and functional groups), and light availability and concentration of elements of the topsoil (0–15 cm) was examined using Pearson correlation at the plot level. Differences between forest types in species richness and exponential of Shannon entropy were examined using Student's t-test. We compared the relative abundance of (1) number of species, (2) number of individuals of understorey plants of different functional groups, and (3) number of individuals of shade-tolerant and shade-intolerant tree seedlings between the two forest types using Chi-square tests. Similar analyses were conducted for the differences among plots of the same forest type and, because no significant differences were found, we pooled the data from all plots for between forest type analyses. Grass species and non-woody vine species had some expected frequencies less than one, which is not appropriate for Chi-square tests, so grass and forb species were merged as ‘herb’ and non-woody vine and liana species were merged as ‘vine’.
Results
Understorey plant diversity
We recorded 63 and 46 understorey plant species in the C. japonica plantation and the natural hardwood forest, respectively. All the understorey plants are native species. Richness of understorey plant species was not significantly different between the forest types, but exponential of Shannon entropy was significantly higher in the C. japonica plantation than the natural hardwood forest (Table 1). In the natural hardwood forest, the three most common understorey species represented ca. 80% of total individuals: Elatostema lineolatum Forst. var. major Thwait (58.5%), Diplazium dilatatum Blume (14.1%), Blastus cochinchinensis Lour (5.2%). The three most common species in the plantation, D. dilatatum (31.2%), E. lineolatum (19.6%) and Lasianthus fordii Hance (10.1%), represented ca. 60% of the total individuals.
| Stand Type | Natural Hardwood Forest | C. japonica | P a |
|---|---|---|---|
| Species Richness | 17.3 ± 4.54 | 21.1 ± 5.60 | 0.22 |
| Exponential of Shannon Entropy | 5.10 ± 0.78 | 8.85 ± 1.13 | 0.02 |
| Proportion of Direct Sunlight | 0.182 ± 0.006 | 0.164 ± 0.050 | 0.005 |
| Proportion of Indirect Sunlight | 0.146 ± 0.002 | 0.138 ± 0.002 | 0.003 |
- a P-value for Student's t-test for the difference in species richness and exponential of Shannon entropy and of nested ANOVA for proportion of direct and indirect sunlight between natural hardwood forest and the C. japonica plantation.
Elatostema lineolatum was the most abundant understorey species in all six natural hardwood forest plots, whereas the plantation plots varied in which species was most abundant. E. lineolatum was the most common species in two of the six C. japonica plots, but it was absent in one plot, and D. dilatatum was the most common species in the other four plots.
Only 18 species occurred in both types of forest, so that Sørensen coefficient of community was 0.33 and percentage similarity was 40%. No C. japonica seedling was recorded in the natural hardwood forest. Seedlings of only five hardwood tree species with a total of 25 individuals were recorded in the C. japonica plantation, as compared to 11 species and 75 individuals under the natural hardwood forest. Only two species of tree seedling, Prunus phaeosticta (Hance) Maxim and Schefflera octophylla (Lour.) Harms, were found in both forest types. For tree seedlings, Sørensen coefficient of community was only 0.13 and percentage similarity was only 6.7%.
In the C. japonica plantation only one tree seedling belonging to Lagerstoemia subcostata Koehne is shade-intolerant, while all others are very shade-tolerant. In the natural forest 23 seedlings (22 Lindera communis Hemsl. and one Michelia compressa (Maxim.) Sargent) are shade-intolerant. The relative numbers of shade-tolerant and shade-intolerant individuals were different between the two forest types (χ2 = 7.31, df = 2, P = 0.07).
The relative numbers of species in different functional groups were not different between the two forest types (χ2 = 5.73, df = 4, P = 0.22; Fig. 1a). In contrast, the relative numbers of individuals in different functional groups were different between the two forest types (χ2 = 257, df = 6, P < 0.001; Fig. 1b).
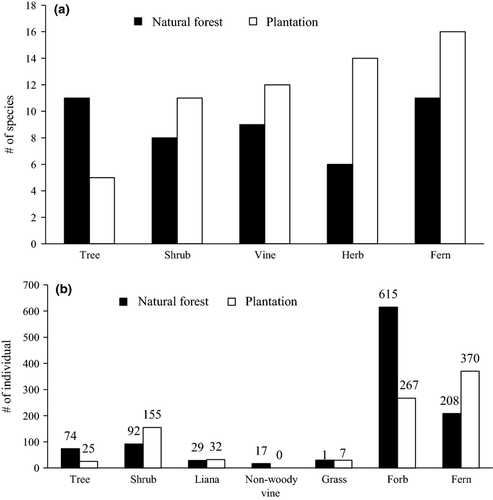
Understorey light environment and plant diversity
Understorey light availability differed both between the two forest types (F = 8.15, P = 0.005 for direct light and F = 9.96, P = 0.002 for indirect light) and among stands of each forest type (F = 6.10, P < 0.001 for direct light and F = 5.87, P < 0.001 for indirect light). Both direct and indirect understorey light availability as proportion of incident sunlight were lower in the C. japonica plantation than the natural hardwood forest (Table 1).
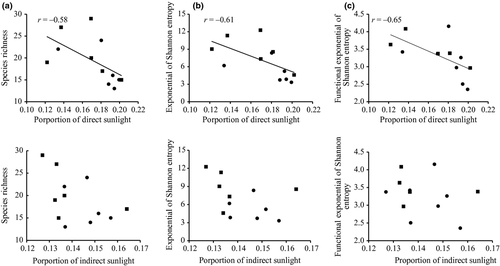
Across the two forest types, species richness was negatively correlated with direct sunlight (r = −0.58, P = 0.049) but not with indirect sunlight (r = −0.49, P = 0.11; Fig. 2a). Similarly, both species diversity and functional diversity (exponential of Shannon entropy) were negatively correlated with direct sunlight (r = −0.61, P = 0.040 for species diversity and r = −0.65, P = 0.021 for functional diversity) but not indirect sunlight (r = −0.41, P = 0.19 for species diversity and r = −0.24, P = 0.45 for functional diversity; Fig. 2b,c).
Gas exchange
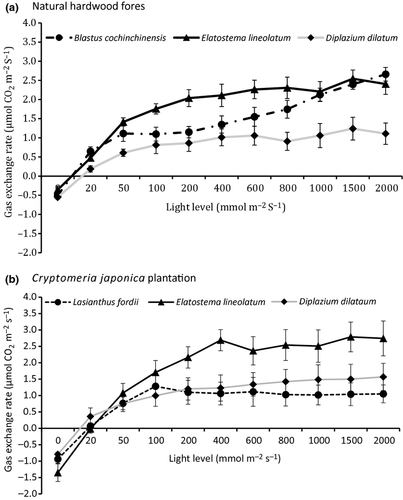
Elatostema lineolatum had the highest CO2 exchange rate among the three most common species in both the hardwood forest and the C. japonica plantation for light levels above 50 mmol·m−2·s−1 (Fig. 3). Light saturation of the three most common understorey species was more prominent in the C. japonica, which occurred approximately at a light level of 400 mmol·m−2·s−1, than the natural hardwood forest (Fig. 3).
Soil pH, element content and cation exchange capacity
Soil CEC and exchangeable concentrations of all analysed elements were significantly higher at the natural hardwood forest than the C. japonica plantation (Table 2). pH did not differ between the forest types (Table 2). Plant diversity was not significantly correlated with nutrient concentrations (N, P, K, Ca, Mg), of topsoil (all P-values > 0.05). pH was correlated with both species richness (r = 0.78, P = 0.004) and exponential of Shannon entropy (r = 0.75, P = 0.008).
| Depth (cm) | Natural Hardwood Forest | C. japonica Plantation | ||||
|---|---|---|---|---|---|---|
| 0–15 | 15–30 | 30–60 | 0–15 | 15–30 | 30–60 | |
| pHn.s., n.s. | 4.35 ± 0.13 | 4.47 ± 0.10 | 4.43 ± 0.07 | 4.13 ± 0.08 | 4.32 ± 0.09 | 4.39 ± 0.08 |
| Total N (g·kg−1)*,* | 3.75 ± 0.57 | 2.68 ± 0.34 | 2.12 ± 0.17 | 2.53 ± 0.08 | 1.85 ± 0.07 | 1.46 ± 0.13 |
| Total C (g·kg−1)*,* | 37.5 ± 8.00 | 25.4 ± 5.40 | 17.3 ± 2.60 | 23.5 ± 0.92 | 14.9 ± 1.06 | 9.84 ± 0.31 |
| P (mg·kg−1)*,* | 38.3 ± 19.4 | 31.2 ± 12.9 | 12.5 ± 2.28 | 18.4 ± 4.80 | 26.0 ± 7.85 | 14.2 ± 2.83 |
| Ca (cmol·kg−1)*,* | 1.82 ± 1.38 | 0.32 ± 0.20 | 0.08 ± 0.04 | 0.09 ± 0.01 | 0.05 ± 0.003 | 0.04 ± 0.005 |
| K (cmol·kg−1)*,* | 0.35 ± 0.09 | 0.22 ± 0.03 | 0.17 ± 0.02 | 0.20 ± 0.02 | 0.17 ± 0.02 | 0.15 ± 0.009 |
| Mg (cmol·kg−1)*, n.s. | 0.51 ± 0.21 | 0.22 ± 0.06 | 0.12 ± 0.04 | 0.14 ± 0.02 | 0.09 ± 0.008 | 0.07 ± 0.009 |
| Na (cmol·kg−1)*, n.s. | 0.09 ± 0.01 | 0.08 ± 0.006 | 0.07 ± 0.005 | 0.07 ± 0.005 | 0.07 ± 0.002 | 0.07 ± 0.004 |
| CEC (cmol·kg−1)*,* | 17.5 ± 2.49 | 12.4 ± 1.76 | 12.2 ± 1.38 | 13.4 ± 0.77 | 11.1 ± 0.56 | 9.22 ± 0.85 |
- n.s.: not significant, *: significantly different at α = 0.05. The first superscript indicates statistical difference between the two forest types and the second label indicate statistical difference among the three depths, based on ANOVA, with depth nested within forest type.
Discussion
Plant species and functional diversity and composition
The higher exponential of Shannon entropy in the monoculture C. japonica plantation than the mixed natural forest rejects our first hypothesis (H1) that predicts the opposite. The result is somehow unexpected and could be explained by the negative relationship between understorey plant diversity and light availability (see below). Nonetheless, it indicates that although monoculture tree plantations lead to low canopy plant diversity, the effects on understorey plant diversity could be different. The low similarity of understorey plant communities between the two forest types supports our second hypothesis (H2), and indicates that the two forests contain very different understorey plant communities, regardless of similar species richness. The differences in understorey plant communities are also evident from the differences in the relative abundance of plants in different functional groups between the two forest types, which support our third hypothesis (H3). We observed species that were near but did not intercept the transect lines in both forest types. Rare small species may be underrepresented due to the use of transect lines instead of belt transects. Yet, there was no evidence from our field observation that this would cause systematic bias (i.e. a larger underestimate) for either of the two forest types that would affect the differences in understorey plant diversity between the two forest types reported in this study.
With only two tree species of the natural hardwood forest having seedlings in the plantation and the very low community similarity of tree seedlings between the two forest types, regeneration of a natural hardwood forest from the abandoned plantation through secondary succession is likely to be a slow process, dependent in part on the eventual mortality of the planted C. japonica cohort. Moreover, the species composition of the eventual regenerated forest will likely be quite different from the adjacent natural forest for some time.
A lack of seed dispersal from surrounding natural hardwood forests could be one important factor leading to the development of distinctive understorey plant communities. Previous studies at the natural forest have indicated that seed dispersal is a major limitation to the recruitment of seedlings, as mean dispersal distance is only 4.7–41.2 m for seven common species, which included two of the seedling species, L. acuminata (21.6 m) and E. roxburghiana (10.5 m), recorded in our plots (Chang-Yang 2013; Chang-Yang et al. 2013). Nonetheless, the higher understorey plant diversity of the C. japonica plantation than the natural hardwood forest suggests that although monoculture forest plantation significantly lowered canopy plant diversity, it might actually promote overall plant diversity, particularly at the landscape scale where it is intermixed with hardwood forests.
Light availability
The significant differences in understorey light availability between the two forests support our fourth hypothesis (H4) and are directly related to forest management. C. japonica were planted at a regular and short interval (2 m) and were likely not artificially thinned prior to the abandonment. The taller canopy, higher DBH and higher stand density in the C. japonica plantation than the natural hardwood forest (Chen 2000) resulted in the development of a more closed canopy and darker understorey. Humidity is higher in the darker understorey of the C. japonica plantation and contributed to its relatively more abundant ferns, relative to the natural forest, as ferns, in general, inhabit more shaded humid micro-environments (Fig. 1; Watkins & Cardelús 2012).
Regardless of these differences, light availability was high under both the C. japonica plantation and the natural hardwood forest (14–18% of full sunlight; Table 1) compared to many mature temperate and tropical forests, which typically have understorey light less than 5% of full sunlight (Canham et al. 1990). Frequent canopy defoliation resulting from typhoon disturbance, on average 1.4 typhoons·yr−1, is key to the high understorey light availability (Lin et al. 2003, 2011). Yet, because the two forests are very close to each other (only 5 km apart) typhoon disturbance is unlikely the cause for the differences in light availability between the two forests.
Although both direct and indirect solar radiation contribute to the overall understorey light availability, they differ qualitatively. The amount of direct sunlight is the dominant control over maximum temperature, relative humidity and available PAR. Studies in both temperate and tropical forests indicate that brief periods of high-intensity direct sunlight (sunflecks) are responsible for much of the daily PAR received by plants growing beneath the canopies of forests (Chazdon & Fetcher 1984; Chazdon 1988; Watling et al. 1997). Because Fushan is often cloudy and it rains very frequently, understorey indirect radiation generally falls between 5–20 μmol·m−2·s−1 in the winter and 5–50 μmol·m−2·s−1 in the summer (Lin 1999). However during sunny days direct radiation often reaches 200–300 μmol·m−2·s−1 in winter and 300–550 μmol·m−2·s−1 in summer, with occasional events as high as 1000 μmol·m−2·s−1 (Lin 1999). Understorey plants probably rely on the high-intensity direct sunlight for much of their photosynthesis because indirect sunlight is often near or below the compensation points of the three most common understorey plants (Fig. 3). A study at Fushan indicates that Alnus formosana, a common understorey species, requires sunflecks for germination (Chang 1996). The study also indicates that wavelengths of indirect sunlight under the forest canopy are predominantly >700 nm (far-red), and this spectral signature likely inhibits seed germination for three pioneer tree species, A. formosana, Trema orientalis (L.) Blume and Broussonettia papyrifera (L.) Vent. (Chang 1996). This qualitative difference between direct and indirect sunlight could explain why understorey plant richness and diversity were more related to availability of direct sunlight than indirect sunlight.
The negative correlations between understorey light availability and plant diversity support our final hypothesis (H5) and the explanation of higher light availability leading to lower plant diversity in the natural hardwood forest. The negative relationship could be explained by ‘competitor inhibition’. Because few species can survive under very low light environments, increasing light availability or spatial variation in light availability, would allow more plant species to co-exist. However, once light availability exceeds a certain level, the vigorous growth of a few species that are highly competitive for light might inhibit the growth of other understorey species. Studies on tree seedling establishment in the Pacific Northwest of the United States indicate that under a high light environment, light competitors such as mosses, herbs and shrubs, could inhibit the growth of seedlings and dominate the forest understorey (Harmon & Franklin 1989; Kennedy & Quinn 2001). A study of seedling distribution and survival at Fushan indicates that seedling density and survival rates are lower under large canopy gaps with vigorous growth of herbaceous plants (Lin 1999).
Elatostema lineolatum is likely a strong light competitor, as suggested by its high photosynthetic rate in both the natural hardwood forest and the C. japonica plantation forest. A study in southern Taiwan has shown that it is a shade-tolerant species with a relatively low photosynthetic light compensation point (4.9 μmol·photon·m2·s−1) but high CO2 use efficiency (0.0088 at CO2 of 360 ppm) compared to co-occurring shade-tolerant plants (mean light compensation point 6.8 μmol·photon·m2·s−1 and mean CO2 use efficiency 0.0069; Cheng & Kuo 2004). Field observation indicates that E. lineolatum occurred in a wide range of light environments, suggesting that it is probably a shade-tolerant species with growth greatly enhanced by high light levels (Fig. 3). The light level under the hardwood forest was likely high enough for vigorous growth of E. lineolatum and suppressed the growth of other plants, including some ferns. In contrast, because the C. japonica plantation had significantly lower sunlight than the natural hardwood forest, the advantage of higher growth rate of E. lineolatum cannot be fully realized. This is likely the reason that it was not the most common species in the C. japonica plantation and the reason for the higher plant diversity under the C. japonica plantation than the natural hardwood forest.
The higher soil fertility indicted by the higher concentration of most nutrient elements of the hardwood forest than the C. japonica plantation (Table 2) may also have contributed to its lower understorey plant diversity. The adverse effect of resource enhancement on diversity has been reported for soil nutrients for several decades (Kirchner 1977; Stevens et al. 2004). For example, diversity of plant communities at Holkham, UK, has been linked to its low nutrient status, and nutrient enrichment adversely affects the diversity (Boorman & Fuller 1982). Nitrogen eutrophication is known to lower plant diversity through competitive exclusion (Bobbink et al. 1998; Stevens et al. 2004). Just as high soil fertility may lead to low plant diversity, high light availability, although it has a positive impact on plant growth, could have a negative effect on plant diversity (Passarge et al. 2006). In low nutrient and/or low light environments, plant populations are often limited by these resources, and therefore more species are able to co-exist. As resource limitation is released, superior competitors would dominate the community, resulting in lower species diversity. Yet, unlike the well-known effects of soil fertility on plant diversity, the negative relationship between understorey light availability and plant diversity is rarely examined.
Conclusions
Our study illustrates a scenario in which replacing natural forests with forest plantations, which were subsequently abandoned, led to differences in understorey light availability, which in turn affected understorey plant diversity and composition. If maintaining species and functional diversity is a goal in managed forest landscapes, effort must be made to assess the status of shade-adapted understorey plants and prevent their loss (Robert & Zhu 2002). Our results also show that although species richness did not differ between the two forest types, the two understorey plant communities had very different species and functional compositions. In terms of secondary succession, because only five hardwood tree species occur in the understorey of the forest plantation 35 yr after abandonment, the recovery of a natural hardwood forest is going to be a slow process, and the community structure could be very different from the adjacent natural forests.
Acknowledgements
The authors wish to thank the Fushan Research Center of Taiwan Forestry Research Institute for logistical support. This study is supported in part by a grant from the National Science Council of Taiwan (NSC 98-2313-B-003-001-MY3). The authors have no conflict of interest and I have confirmation from all co-authors on this submission.