ceRNA Profiling Reveals circSAMD4A Promoted Porcine Adipocytes Differentiation via Targeting miR-127/PRKAR2B
Funding: This work was supported by the Sichuan Province Science and Technology Support Program (2022NSFSC1778, 2022YFH0063, and 2022YFH0064) and the Open Fund of the Farm Animal Genetic Resources Exploration and Innovation Key Laboratory of Sichuan Province (CNDK-2021-03).
Zhenzhen Zhang and Huali Chen contributed equally to this work and should be considered cofirst authors.
ABSTRACT
Pork quality is critically influenced by fat deposition patterns, with emerging evidence implicating circular RNAs (circRNAs) as key regulators of adipogenesis, though the specific circRNAs controlling lipogenesis remain incompletely characterized. To systematically elucidate the competing endogenous RNA (ceRNA) network governing porcine adipocyte differentiation, we isolated primary preadipocytes from piglets, induced differentiation, and performed comprehensive RNA sequencing that identified 504 differentially expressed (DE) circRNAs, 126 DE miRNAs, and 1615 DE mRNAs between proliferative and differentiated states, revealing an extensive transcriptomic rewiring during adipogenesis. Functional characterization demonstrated that two circRNAs, circSAMD4A and circLPAR1, significantly promoted lipid accumulation. The further mechanistic studies established that circSAMD4A functions by competitively binding miR-127, alleviating the repression of PRKAR2B, thereby enhancing adipogenic differentiation. These findings not only advance fundamental understanding of circRNA biology in adipose tissue but also provide actionable molecular targets for precision breeding strategies aimed at optimizing intramuscular fat deposition.
1 Introduction
Globally, pigs play a vital role as a primary meat source, with pork accounting for over 30% of total meat consumption in 2022 (FAOSTAT, https://www.fao.org/faostat/zh/#data/QL). Extensive studies have established a strong relationship between adipose tissue and key meat production traits, including yield and quality (Chen et al. 2021). Economically important characteristics such as backfat thickness, carcass leanness, and feed efficiency are closely tied to fat deposition patterns (Schumacher et al. 2022). Given these associations, understanding the genetic mechanisms governing fat deposition is essential for developing effective breeding strategies that enhance both production efficiency and meat quality.
Adipogenesis, the differentiation of preadipocytes into mature adipocytes, critically influences pork quality through its regulation of fat deposition characteristics, including content, distribution, and composition (Yi et al. 2023). Intramuscular fat (IMF) content serves as the primary quality determinant, where optimal levels (typically 2%–4%) significantly enhance tenderness, juiciness, and flavor by improving texture refinement and aroma development, while excessive deposition leads to undesirable greasiness. The spatial distribution of IMF, particularly when forming marbling patterns, substantially improves palatability. The fatty acids (FAs) in fat play a pivotal role, as higher proportions of unsaturated FAs improve tenderness and health benefits, and excessive unsaturation may compromise oxidative stability and shelf life. Additionally, the chain length and degree of saturation of FAs determine the melting point and hardness, which critically influence both processing characteristics and sensory mouthfeel. The adipogenesis process itself is regulated by an interplay of genetic factors, nutritional components, and management practices.
The adipogenesis process is orchestrated by a tightly controlled transcriptional cascade and signaling pathways (Audano et al. 2022; Liu et al. 2022). Key regulators include the CCAAT/enhancer-binding protein (C/EBP) family, particularly C/EBPα, C/EBPβ, and C/EBPδ, which activate peroxisome proliferator-activated receptor gamma (PPARγ), a master transcription factor governing adipogenesis. The induction of PPARγ subsequently upregulates adipocyte-specific genes such as fatty acid synthase (FAS), fatty acid-binding protein (FABP), and hormone-sensitive lipase (HSL) (Song et al. 2020). Beyond transcriptional regulation, adipogenesis is further modulated by post-transcriptional mechanisms, including noncoding RNAs (ncRNAs) (Ru et al. 2023). A deeper understanding of these molecular mechanisms can inform strategies to optimize fat deposition, thereby enhancing both production efficiency and meat quality in swine breeding programs.
Recent advances in high-throughput sequencing and molecular biology have revealed an extensive repertoire of noncoding RNAs (ncRNAs), including circular RNAs (circRNAs), long non-coding RNAs (lncRNAs), and microRNAs (miRNAs), which play critical roles in adipose tissue biology. circRNAs, formed through back-splicing of mRNA transcripts, have emerged as key regulators of adipocyte development and function (Lasda and Parker 2014; Zhang et al. 2022). These molecules exert their regulatory effects through multiple mechanisms, such as functioning as miRNA sponges, modulating alternative splicing, interacting with RNA-binding proteins (RBPs), and even encoding functional peptides in some cases (Huang et al. 2020).
Growing evidence demonstrates that numerous circRNAs containing miRNA binding sites participate in adipogenesis regulation via competitive endogenous RNA (ceRNA) networks. In humans, circSAMD4A promotes preadipocyte differentiation by sponging miR-138-5p to upregulate EZH2 expression (Liu et al. 2020). In cattle, circFUT10 inhibits adipogenesis by sequestering let-7 miRNA, leading to increased PPARGC1B levels (Jiang et al. 2020). While in pigs, circSETBP1 accelerates adipocyte differentiation through miR-149-5p sponging (Liu et al. 2022). Additional porcine studies have identified circPPARA as a positive regulator of intramuscular preadipocyte differentiation (Li et al. 2022), and bovine research has shown circBDP1 enhances preadipocyte proliferation and differentiation (Zhang et al. 2022). Despite these significant findings, research on circRNA-mediated regulation of adipogenesis remains in its infancy. Many circRNAs involved in adipose tissue development await discovery, and their precise molecular mechanisms require further elucidation. The expanding catalog of adipogenic circRNAs underscores their potential as novel therapeutic targets and molecular markers for metabolic disorders and meat quality improvement.
Therefore, this study employed transcriptomic profiling to systematically characterize the circRNA–miRNA–mRNA regulatory network governing porcine adipocyte differentiation. We aimed to identify differentially expressed (DE) circRNAs during adipogenesis, screen and functionally validate candidate circRNAs regulating lipid accumulation, and mechanistically dissect how these circRNAs modulate adipogenesis through ceRNA networks.
2 Materials and Methods
2.1 Primary Porcine Adipocyte Isolation and Culture
Primary porcine preadipocytes were isolated according to established protocols (Gao et al. 2019). Briefly, subcutaneous adipose tissue samples from newborn piglets were minced and digested with 0.1% Type I collagenase in PBS at 37°C under continuous stirring for 60 min. The resulting cell suspension was filtered through a 200-μm mesh to remove undigested debris, which was centrifuged at 300 × g for 10 min subsequently. Erythrocytes were lysed using ammonium chloride solution. Finally, the isolated cells were resuspended, plated in culture dishes, and maintained at 37°C in a humidified 5% CO2 atmosphere.
Cells were cultured in growth medium (GM) containing DMEM/F12 supplemented with 10% fetal bovine serum (FBS, Gibco, New York, NY, USA) until reaching 80% confluence (Day −2). Adipogenic differentiation was induced by replacing GM with differentiation medium (DM) for 48 h until complete cell fusion (Day 0). The DM consisted of GM supplemented with 0.5-mM 3-isobutyl-1-methylxanthine (IBMX), 1-nM dexamethasone, and 5 μg/mL insulin. Cells were maintained in DM until mature adipocytes are formed, with medium changes every 48 h to ensure complete differentiation.
All animal procedures were performed in accordance with the guidelines approved by the Animal Ethics Committee of Southwest University of Science and Technology (Approval No. L2022021).
2.2 The RNA Sequencing for circRNAs, miRNAs, and mRNAs
The preadipocytes and the mature adipocytes were collected for high-throughput sequencing (n = 2). RNA sequencing was conducted by the Beijing Genome Institute (BGI, Shenzhen, China) using the BGISEQ-500 platform. Libraries for circRNA, miRNA, and mRNA were constructed following previously established protocols (Bao et al. 2020; Fehlmann et al. 2016; Zhu et al. 2018). The sequencing data underwent quality filtration to generate clean reads, which were subsequently aligned to the reference genome (Sus scrofa) using HISAT2. Transcript assembly and quantification were performed using Stringtie. For small RNA analysis, clean reads were mapped to the Sus scrofa genome using Bowtie2 and quantified with FeatureCounts. circRNA prediction was carried out using CIRI and find_circ software (Gao et al. 2015; Memczak et al. 2013), and the union set of predicted circRNAs was retained for further analysis. The raw sequencing data have been deposited in the NCBI Sequence Read Archive under accession numbers PRJNA1055429 and PRJNA1062518.
2.3 Differential Gene Analysis and Functional Enrichment Analysis
DESeq2 was utilized for the differential expression (DE) analysis of circRNAs, miRNAs and mRNAs. The false discovery rate (FDR) was calculated by adjusting the p value with the Benjamini–Hochberg method. DE genes were identified based on an FDR threshold of ≤ 0.05 and an absolute log2 fold change (|log2FC|) threshold of ≥ 1. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed using the DAVID Conversion Tool (https://david.ncifcrf.gov/conversion.jsp).
2.4 ceRNA Network Construction
We computationally predicted RNA interactions using stringent bioinformatic approaches. circRNA-miRNA interactions were identified using miRanda with a score cutoff ≥ 140, while miRNA-mRNA interactions were predicted through complementary algorithms (miRanda score ≥ 140 and RNAhybrid with minimum free energy ≤ −20 kcal/mol plus p value ≤ 0.05). Integrated coexpression analysis of circRNA, miRNA and mRNA expression profiles enabled construction of the ceRNA network, which was visualized and analyzed using Cytoscape (https://cytoscape.org/). This dual-algorithm approach with rigorous thresholds ensured high-confidence interaction predictions for subsequent network analysis.
2.5 The Transfection of Adipocytes
Full-length sequences of circSAMD4A, circVCAN, circLPAR1, and circWWP1 were commercially synthesized and cloned into the pLCDH-ciR vector (Geneseed, Shanghai) to generate overexpression constructs, while miR-127 mimics and PRKAR2B-specific siRNA were designed and obtained from GenePharma (Shanghai). For functional assays, preadipocytes at 60% confluence were transfected with individual circRNA overexpression vectors or cotransfected with either miR-127 mimics or siPRKAR2B using X-tremeGENE HP DNA Transfection Reagent (Roche, Mannheim, Germany), followed by standard adipogenic differentiation induction as previously described.
2.6 cDNA Synthesis and Real-Time Quantitative PCR (qPCR)
Total RNA was extracted from cells and reverse transcribed into cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (Takara, Dalian, China), with parallel miRNA-specific reverse transcription employing stem-loop primers (Che et al. 2024; Zhang et al. 2018). Quantitative PCR analysis was conducted using TB Green Premix Ex Taq II on a CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA), with expression levels normalized to 18s rRNA (circRNAs and mRNAs), and U6 snRNA (miRNAs). Relative quantification was calculated using the 2−ΔΔCt method, with specific divergent primers (Table S1) ensuring accurate transcript detection.
2.7 Western Blot
Western blot analysis was performed according to established protocols (Zhang et al. 2019) using primary antibodies against FAS (4233T), FABP4 (50699S), PPARγ (2435T), HSL (4107S), and β-actin (2146S) from Cell Signaling Technology (Danvers, MA), with corresponding rabbit secondary antibodies obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Protein samples were separated by SDS-PAGE, transferred to PVDF membranes, and probed with antibodies diluted according to manufacturers' specifications, followed by chemiluminescent detection to quantify expression levels of target proteins.
2.8 Boron-Dipyrromethene (BODIPY) Fluorescent Dye Staining
The staining procedure was performed according to the manufacturer's protocol (Beyotime Biotechnology, Shanghai, China), with cell nuclei specifically labeled using Hoechst 33342 (2 μg/mL).
2.9 RNA Pull-Down Assay
RNA pull-down assays were performed to investigate miRNA-circRNA interactions using the RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, Cleveland, OH, USA). Biotin-labeled miR-127, miR-103, and negative control miR-NC were transfected into 293T cells, followed by cell lysis and incubation with streptavidin-coated agarose beads to capture RNA-protein complexes. The specifically bound circSAMD4A and circLPAR1 were then eluted and quantified by qPCR using circRNA-specific divergent primers, with mock-treated samples serving as controls to verify interaction specificity.
2.10 Reporter Constructs and Dual-Luciferase Reporter Assay
We conducted luciferase reporter assays to validate the target of miR-127 by cloning the predicted binding region into the pGL3-basic vector (Promega); 293T cells were cotransfected with the constructed reporter, miR-127 mimics, and Renilla luciferase control plasmid (pRL-TK) using Lipofectamine 3000. After a 48-h incubation, dual-luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
2.11 Data Analysis
All experimental data are presented as mean ± SEM (standard error of mean) from at least three independent biological replicates. Statistical significance was determined using one-way ANOVA with post hoc Tukey's test in SPSS (v26.0, IBM), with significance thresholds set at *p < 0.05, **p < 0.01, and ***p < 0.001.
3 Results
3.1 The Notable Differences of Circular RNA Profile in Porcine Preadipocytes and Mature Adipocytes
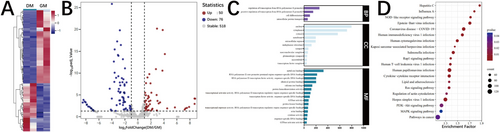
The RNA sequencing identified 8453 distinct circRNAs across all samples, with exon-derived circRNAs representing > 94% of the total (Figure 1A, Table S2). Comparative quantification revealed 504 DE circRNAs with 258 upregulated and 246 downregulated in DM compared with GM (Figure 1B–D), including 15 most significantly altered candidates (Table S3). Functional annotation of their host genes showed significant enrichment in adipogenesis-relevant processes, such as ubiquitin-mediated proteolysis, cellular localization, ATP binding, and kinase/ligase enzymatic activities (Figure 1E). Pathway analysis further implicated DE circRNAs in key adipogenic signaling cascades including TGF-β, FoxO, thyroid hormone, and relaxin pathways (Figure 1F), which are known to regulate adipocyte differentiation (Kopchick et al. 2020; Lee et al. 2019; Obregon 2008). These comprehensive findings establish circRNAs as important molecular players in porcine adipogenesis through their potential regulation of both metabolic processes and developmental signaling pathways.

3.2 The DE miRNA Profile Between Porcine Preadipocytes and Mature Adipocytes
Building upon established roles of circRNAs as miRNA sponges (Jarlstad Olesen and L 2021), we comprehensively characterized the miRNA transcriptome during porcine adipocyte differentiation. Our sequencing identified 644 miRNAs (354 known and 290 novel), with 126 DE (50 upregulated, 76 downregulated) in mature versus preadipocytes (Figure 2A,B). Target prediction using RNAhybrid and miRanda algorithms revealed that DE miRNA targets were significantly enriched in adipogenesis-relevant processes, including regulation of transcription from RNA polymerase II promoter, cell differentiation, identical protein binding, and protein kinase binding (Figure 2C). Pathway analysis further implicated these miRNAs in critical signaling cascades including NOD-like receptor, Rap1/Ras, PI3K-Akt, and MAPK pathways (Figure 2D). These findings collectively demonstrate that miRNAs serve as crucial regulators of porcine adipogenesis through their coordinated modulation of both transcriptional networks and key signaling pathways.
3.3 The DE mRNA Analysis in Porcine Adipocytes Before and After Differentiation
RNA sequencing analysis identified 964 upregulated and 651 downregulated mRNAs during porcine adipocyte differentiation (Figure 3A). Functional enrichment analysis demonstrated these mRNAs were significantly associated with the processes of immune regulation, cellular differentiation, and signal transduction mechanisms (Figure 3B). Notably, pathway analysis demonstrated the conserved involvement of the PI3K-Akt, MAPK, cAMP, and calcium signaling pathways, which aligns with the functional patterns observed in both circRNA and miRNA profiles (Figure 3C). This finding suggests coordinated regulation among all three RNA species during adipogenesis. These results establish mRNA expression dynamics as integral components of the multilayered regulatory network governing porcine fat cell development.
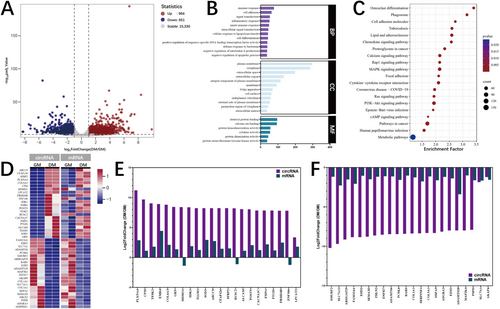
To elucidate the relationship between circular and linear RNA dynamics during adipocyte differentiation, we systematically compared expression patterns of GE circRNAs and their cognate linear transcripts (Figure 3D). While most circRNAs exhibited concordant expression trends with their host genes (Figure 3E,F), suggesting coregulation through shared transcriptional mechanisms. However, a small number of circRNA, such as circMSMO1 (derived from methylsterol monooxygenase 1, MSMO1), circRUSC2 (derived from RUN and SH3 domain containing 2, RUSC2), and circZNF106 (derived from zinc finger protein 106, ZNF106), displayed significant divergence from their linear counterparts (Figure 3E).
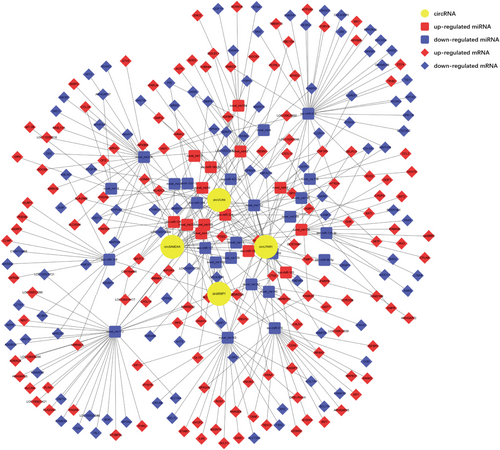
3.4 The circRNA–miRNA–mRNA Network in Regulating Porcine Adipogenesis
Through systematic integration of these data, we constructed a comprehensive competing endogenous RNA (ceRNA) network using Cytoscape to elucidate the coordinated regulation of circRNAs, miRNAs, and mRNAs during adipocyte differentiation. The network centers on four functionally relevant circRNAs, circSAMD4A (derived from sterile alpha motif domain containing 4A, SAMD4A), circVCAN (derived from versican, VCAN), circLPAR1 (derived from lysophosphatidic acid receptor 1, LPAR1), and circWWP1 (derived from WW domain containing E3 ubiquitin protein ligase 1, WWP1), which collectively interface with 39 DE miRNAs (14 upregulated, 25 downregulated) and 240 target mRNAs (136 upregulated, 104 downregulated) (Figure 4).
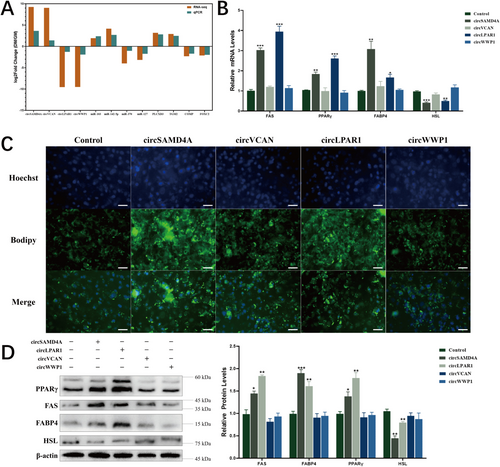
3.5 circSAMD4A and circLPAR1 Promoted Porcine Adipocytes Differentiation
qPCR validation confirmed RNA-seq expression patterns of circSAMD4A, circVCAN, circLPAR1, circWWP1, miR-103, miR-142-5p, miR-370, miR-127, phosphatidylinositol-specific phospholipase C X domain containing 3 (PLCXD3), transglutaminase 2 (TGM2), cartilage oligomeric matrix protein (COMP), and forkhead box C2 (FOXC2) levels were consistent with RNA-seq (Figure 5A). To further investigate the roles of circRNAs in porcine adipocyte differentiation, we constructed overexpression vectors for circSAMD4A, circVCAN, circLPAR1, and circWWP1. Functional characterization revealed distinct adipogenic roles. circSAMD4A and circLPAR1 significantly upregulated the lipogenic markers FAS, PPARγ, and FABP4 while suppressing the lipolytic marker HSL at both the mRNA (Figure 5B) and protein levels (Figure 5D). In contrast, circVCAN and circWWP1 exhibited negligible effects on these markers. Complementary Hoechst staining demonstrated that only circSAMD4A and circLPAR1 enhanced lipid droplet accumulation (Figure 5C). These collective results provide evidence that circSAMD4A and circLPAR1 as bona fide regulators of porcine adipogenesis.
3.6 circSAMD4A Targets miR-127/PRKAR2B to Promote Porcine Adipogenesis
Moreover, bioinformatics prediction identified miR-127 and miR-103 as putative binding partners for circSAMD4A and circLPAR1, respectively. RNA pull-down assays validated this interaction for circSAMD4A, showing significant enrichment in miR-127-captured fractions versus mutant controls (Figure 6A), while circLPAR1 failed to demonstrate miR-103 binding (Figure 6B). Subsequent target screening revealed PRKAR2B as a miR-127 target, containing a conserved binding site in its 3′ UTR (Figure 6C). Luciferase reporter assays in 293T cells confirmed this regulatory relationship, showing 60% reduction in PRKAR2B reporter activity with miR-127 cotransfection, and significant rescue of this suppression upon circSAMD4A introduction (Figure 6D). These results establish a complete circSAMD4A/miR-127/PRKAR2B regulatory axis in lipid metabolism.
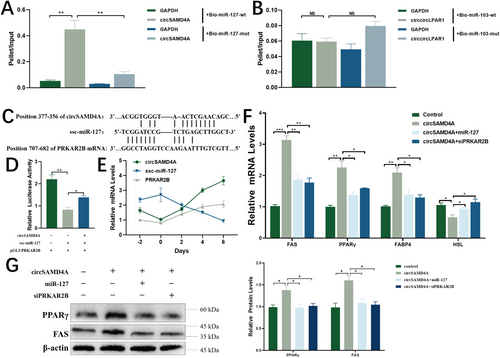
The expression analysis during porcine adipocyte differentiation revealed coordinated regulation within the circSAMD4A-miR-127-PRKAR2B axis, with circSAMD4A and PRKAR2B showing similar temporal patterns that were inversely correlated with miR-127 expression (Figure 6E). Functional perturbation experiments demonstrated circSAMD4A overexpression significantly increased PRKAR2B while decreasing miR-127 levels (Figure S2A), whereas miR-127 overexpression suppressed PRKAR2B without affecting circSAMD4A expression (Figure S2B). Rescue experiments further showed that cotreatment with either miR-127 mimics or PRKAR2B siRNA completely reversed the proadipogenic effects of circSAMD4A overexpression, specifically abrogating its induction of FAS and PPARγ while restoring HSL expression and normalizing FABP4 mRNA levels (Figure 6F,G). These functional assays provide conclusive evidence that circSAMD4A promotes adipogenesis through a linear regulatory pathway involving miR-127 sequestration and subsequent PRKAR2B derepression, ultimately modulating key adipogenic markers including FAS, PPARγ, FABP4, and HSL, thereby establishing the circSAMD4A-miR-127-PRKAR2B axis as a bona fide regulatory module in porcine adipocyte differentiation.
4 Discussion
Adipogenesis is a complex and not yet fully elucidated process characterized by a series of gene activation events that are intricately regulated by numerous factors, many of which remain only partially elucidated. Previous research into the molecular mechanisms of adipogenesis has primarily focused on analyzing DNA, mRNA, and miRNA levels. However, recent studies have revealed that circRNAs play a pivotal role in modulating cellular metabolism (Hansen et al. 2013; Memczak et al. 2013). The development of high-throughput transcriptional profiling methodologies has facilitated the identification of circRNAs across various species, some of which have demonstrated significant involvement in animal growth and development. Recent studies have elucidated circRNA expression patterns in adipose tissue of pigs (Li et al. 2018), mice (Arcinas et al. 2019), humans (Xu et al. 2017), buffalo (Huang et al. 2019), and yaks (Zhang et al. 2020). Nevertheless, a comprehensive understanding of the molecular mechanisms underlying the role of circRNAs in this biological process remains a subject requiring further investigation.
In order to comprehensively examine the functions of circRNAs in porcine adipogenesis, we conducted RNA-seq analysis to characterize the circRNAs profile in primary porcine adipocytes cultured in vitro, thereby identifying a total of 8453 circRNAs. Previous studies have indicated that circRNAs can serve as competitive endogenous RNAs to sequester miRNA molecules, thereby exerting regulatory control over downstream genes. Subsequent investigations revealed the presence of 644 miRNAs and 16,945 mRNAs in the adipocytes. A comparative analysis of their expression levels between preadipocytes and adipocytes identified 504 DE circRNAs, 126 DE miRNAs, and 1615 DE mRNAs. Notably, circSAMD4A and circLPAR1 were found to enhance lipid accumulation in porcine adipocytes, prompting further exploration of its downstream targets.
circRNAs represent a unique class of ncRNAs characterized by their covalently closed circular structure formed through back-splicing of exons, exhibiting tissue-specific and developmental stage–specific expression patterns that make them promising biomarkers for cellular differentiation processes (Misir et al. 2022; Zhou et al. 2018). Our analysis revealed distinct adipogenic expression profiles among DE circRNAs, with circSAMD4A showing adipocyte-specific induction (absent in preadipocytes) while circLPAR1 displayed an inverse pattern—particularly noteworthy given circLPAR1's established role in promoting osteogenesis via hsa-miR-31 sponging in human dental pulp stem cells (Xie et al. 2020), suggesting potential lineage-specific functions across mesenchymal differentiation pathways. The evolutionary conservation of circSAMD4A (96% human–porcine sequence similarity) and its consistent proadipogenic role in both species (Liu et al. 2020; Wei et al. 2020; Xie et al. 2020) strongly supports its fundamental regulatory function in adipocyte development. Our findings extend previous work by mechanistically demonstrating the activity of circSAMD4A through the miR-127/PRKAR2B axis in porcine adipogenesis, reinforcing the growing recognition of circRNAs as critical, conserved regulators of cellular differentiation with potential applications as developmental biomarkers and therapeutic targets.
Emerging evidence has firmly established that select circRNAs function as ceRNAs through their capacity to sequester and inhibit miRNA activity, thereby modulating critical biological processes including adipogenesis (Elkhawaga et al. 2023). Our study focused on the interaction of circSAMD4A and miR-127, a multifunctional miRNA documented to promote myogenesis while suppressing adipocyte differentiation (Gao et al. 2019; Zhai et al. 2017) that is abundantly expressed in adipose tissue. We demonstrate that circSAMD4A contains a conserved binding site for miR-127 and functions as an efficient molecular sponge, effectively titrating miR-127 availability and consequently relieving its repression of proadipogenic targets. This ceRNA mechanism represents a precise regulatory switch wherein circSAMD4A-mediated miR-127 inhibition creates permissive conditions for adipocyte differentiation, providing novel insights into the complex post-transcriptional networks controlling mesenchymal cell fate determination.
PRKAR2B has been established as the dominant regulatory subunit of PKA in adipocytes across multiple species, with demonstrated functional significance in both human and murine models. During fetal adipose development, PRKAR2B serves as the principal PKA regulatory isoform, with knockout studies revealing its metabolic importance. PRKAR2B-deficient mice exhibit elevated metabolic activity through compensatory upregulation of PRKAR1A (Cummings et al. 1996). Clinical observations further underscore its metabolic relevance, showing inverse correlations between PRKAR2B expression in human adipose tissue and both BMI and circulating insulin levels (Mantovani et al. 2009). Mechanistically, PRKAR2B has been identified as the predominant regulatory subunit during adipogenesis in both mouse and human preadipocytes (Peverelli et al. 2013), a finding corroborated by our porcine model demonstrating PRKAR2B upregulation during lipogenesis. Our study experimentally validated PRKAR2B as a direct target of the adipogenesis-inhibiting miR-127, identifying circSAMD4A as a functional miR-127 sponge that alleviates this repression.
Our findings indicate that the targeted modulation of the circSAMD4A/miR-127/PRKAR2B axis constitutes a promising molecular strategy for optimizing IMF deposition, a critical determinant of pork quality, via precision breeding or nutritional interventions. However, it is important to note that the in vitro adipocyte model may not fully replicate the complexity of in vivo metabolism. Future studies should therefore validate these effects in growing pigs under production conditions. Additionally, the potential off-target effects of circRNA manipulation warrant careful evaluation, as constitutive overexpression could disrupt other metabolic pathways. Furthermore, the economic feasibility of genetic versus nutritional approaches requires comparative assessment, particularly with respect to cost–benefit ratios for small-scale producers. Collectively, these findings represent a significant advancement toward precision pork production, contingent upon addressing these practical constraints through targeted follow-up studies.
In conclusion, our integrated multiomics investigation revealed extensive transcriptomic remodeling during porcine adipocyte differentiation, identifying 504 DE circRNAs, 126 miRNAs, and 1615 mRNAs that collectively orchestrate adipogenesis. Functional characterization established circSAMD4A and circLPAR1 as positive regulators of lipid accumulation, with mechanistic studies elucidating that circSAMD4A promotes adipogenesis through a defined ceRNA network, competitively binding miR-127 to alleviate its repression of PRKAR2B. These findings represent a significant advance in understanding the molecular regulation of porcine adipogenesis while outlining a roadmap for developing precision agriculture solutions to improve pork quality through targeted manipulation of circRNA-mediated regulatory networks.
Acknowledgments
This work was supported by the Sichuan Province Science and Technology Support Program (2022NSFSC1778, 2022YFH0063 and 2022YFH0064) and the Open Fund of Farm Animal Genetic Resources Exploration and Innovation Key Laboratory of Sichuan Province (CNDK-2021-03).
Conflicts of Interest
The authors declare no conflicts of interest.




